Expanding Cas9 Enhances Precision in Base Editing
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
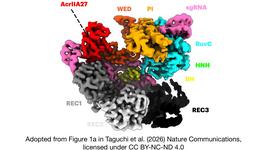
The REC domain is a non-catalytic region of Cas9 responsible for DNA recognition and guide RNA (gRNA) interaction. It helps stabilise the RNA-DNA complex and influences the enzyme’s specificity and efficiency. Unlike the RuvC and HNH domains, which cleave DNA, the REC domain ensures proper target alignment.
To construct GS-Cas9, the researchers inserted an extended REC sequence derived from Streptococcus thermophilus Cas9 (St1Cas9) into specific positions within SpCas9’s REC domain. The insertions were linked using flexible peptide sequences from Staphylococcus aureus Cas9 (SaCas9) to maintain structural integrity. The resulting GS-Cas9 had the largest REC domain experimentally characterised to date, expanding from 624 to 1036 amino acids.
The expanded GS-Cas9 maintains on-target editing while significantly reducing unintended modifications. Additionally, the modification regulates the activity of the TadA8e deaminase in ABE8e, lowering RNA off-target effects and improving editing precision.
By demonstrating a method inspired by natural Cas9 evolution, the research suggests that domain expansion can serve as an alternative strategy for enhancing gene editors beyond traditional mutational approaches.
The study was published in Nature Communications on 28 February 2025.
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.