First of its kind CRISPR-based clinical trial targeting metastatic tumours of the GI-tract

Researchers at the Masonic Cancer Center at the University of Minnesota (UMN) have opened a new clinical trial that utilises CRISPR technology to develop a treatment for metastatic gastrointestinal solid tumour cancers. This phase I/ll clinical trial is the result of new promising research from National Cancer Institute, the sponsors of the clinical trial Intima Bioscience, and UMN on a new T cell therapy for solid tumours, in which CRISPR is deployed to knock out a gene called cytokine-inducible SH2 (CISH).
CISH is a novel cancer immune checkpoint. Unlike the widely discussed checkpoint protein PD-1 that resides on the cell surface, CISH is expressed intracellularly and biophysically proximate to the T cell receptor (TCR) signalling complex. CISH is believed to play a key role in preventing T cells from recognising and eliminating tumours via enhancement of neoantigen reactivity.
The idea behind the new trial, which is supported by promising efficacy in preclinical cancer models and by data in human T cells neoantigen reactive against the patient’s specific cancer mutation, is that CISH inactivation in tumour-infiltrating lymphocytes (TILs) and other T cells will augment their ability to target and destroy solid tumours. Since the CISH checkpoint is not easily druggable with a cell surface-targeting antibody, CRISPR is now used to target this protein, as described in our recent interview with the researchers behind this work.
First Trial on Solid Tumours
The trial is the first of its kind in several aspects; it is the first time CRISPR is used to knock out CISH for therapeutic purposes, and it’s also the first to utilise a CRISPR-engineered T cell therapy to treat metastatic gastrointestinal cancer. While different variations of gene-edited cell therapy have shown to be efficient in a number of haematological cancers, no cell therapy has so far shown the same promising effects in solid tumours. Therefore, this new trial will shed light on whether gene-editing cell therapy can be breakthrough for solid tumour cancers.
»Despite the challenges of starting a clinical trial of this complexity in the middle of the COVID-19 pandemic, we felt an obligation to our patients to bring this novel and promising therapy to the armamentarium to fight solid tumour cancers as soon as possible,« says Gastrointestinal Oncologist Dr. Emil Lou, Associate Professor of Medicine, Division of Hematology, Oncology and Transplantation at the Medical School of University of Minnesota in a press release.
A Complex Clinical Trial
To learn more about the trial, CRISPR Medicine News interviewed Dr. Emil Lou.
»CRISPR is a novel form of gene editing, which can be used to knock out any number of targets. The real centrepiece to this particular trial is our focus on knocking out CISH very specifically, and with extremely high efficiency and specificity (absence of off-target CRISPR editing) in tumour infiltrating lymphocytes (TIL). Non-engineered TILs themselves have been evaluated on many occasions, but combining the power of neoantigen enrichment and efficient CRISPR editing, with focus on this specific target, is a strategy that we found extremely promising, and is the central premise of this trial,« Dr. Lou says.
Clinical haematologists who work with haematological tumours are gradually becoming more familiar with TIL therapy, where the patient’s naturally occurring T cells are harvested from bone marrow, expanded and infused back into the patient. Gene editing therapy in solid tumours requires a much longer and more complex process.
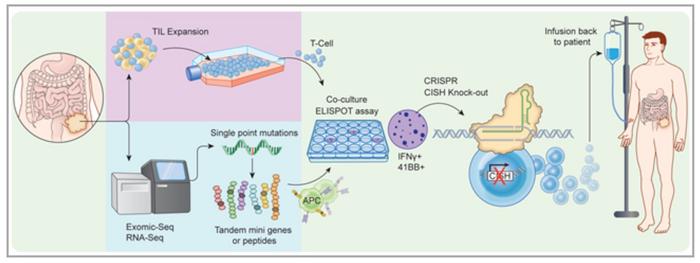
In brief, for this trial, patients have to undergo tumour resection surgery at the M Health Fairview University of Minnesota Medical Center in Minneapolis, where the trial is carried out. Immediately after this, the tumour tissue is sent to a lab to harvest patient T cells. These cells are then subjected to CRISPR, specifically to knock out CISH. Engineered cells are then expanded in numbers in lab culture, and then and reinfused back into the patient. After this, the patients must be carefully followed with scans every month for the first few months, and then at longer intervals after that, to assess the response of their tumours to the engineered TILs.
For each patient it will take approximately three to six months from the recruitment, the surgery and healing, to the actual therapy and getting the first results.
»The overall timeline and design of the trial is, from a practical perspective, another angle that makes this trial more unique and unusual than most clinical trials for cancer. In many cases, when patients have exhausted available standard treatments, they begin receiving the treatment soon after, as they need to be on some form of treatment to prevent further progression. However, this trial works differently in that aspect. It takes several weeks to prepare the TILs in order to give the patient the intended final product for therapy,« says Dr. Lou.
First Step: Recruiting Patients
Gastrointestinal cancer (any cancer from mouth to anus, with a specific burden and focus on colon cancer) is often only discovered at a late stage, when metastasis has already occurred, often to other organs such as the liver or the lung. This means that a large proportion of patients are in fact diagnosed with stage IV or incurable cancer.
The trial enrols patients with stage IV gastrointestinal cancer who would normally only receive palliative treatments at this stage of disease. However, as mentioned earlier, for this trial the patients need not be exhausted by prior chemotherapeutic and/or surgical attempts. Part of the reason for this approach is that patients must be strong enough to tolerate the lymphodepleting chemotherapy that is needed prior to the investigational therapy. Lymphodepletion reduces the patient’s own T cell numbers, so they will not antagonise the proliferation of the infused engineered T cells.
»We are trying to find patients who are relatively healthy, active, and doing well. And some patients are doing very well and even thriving despite metastatic stage IV cancer, which in general is considered incurable. Some of my patients will generally be quite physically active. They have stage IV cancer, but live very well with the disease, and they are prototype candidates for the trial,« Dr. Lou says.
Since the trial opened, there have been many referrals and inquiries from patients, or their physicians, nationally and internationally who are interested in enrolling in the trial. Dr. Lou and his team screen the list of all enquiries to identify patients who may fit best with the trial eligibility.
Communication is essential to carrying out this logistically complex trial
»Our center has several advantages for carrying out a trial like this,« Dr. Lou says.
»We are a National Cancer Institute (NCI)-designated Comprehensive Cancer Center, one of 71 in the U.S. Much of the basic research in CRISPR gene editing of CISH was done here, in collaboration with scientists at the NCI. Besides that, UMN holds one of the five NCI-designated molecular and cellular therapeutic facilities in the US, meaning that clinical-grade cellular therapy products can be manufactured on site. All that, along with us at the Medical School having a well-developed clinical trial infrastructure, put us in a position to carry out the entire trial completely under our own roof, so to speak. And that proximity helps with communication and arranging logistics, because this is definitely a more complicated trial than most,« Dr. Lou says.

The complexity of the trial does not only come down to the infrastructure; many different areas of expertise are needed. It includes physicians in diverse fields of surgery, oncology and bone marrow transplantation. Add to that the basic scientists who understand the CRISPR technology and the T-cell immunology experts. Finally, a key component is also the people who know how to manufacture molecular and cellular therapeutics.
»This effort really requires a lot of teamwork and interdisciplinary coordination. And to coordinate a larger team than usual requires a high level of effective and constant communication. It has been very gratifying to be able to bring in these people from different corners of our university in partnership with Intima Bioscience to make this trial a reality. Ordinarily, they would not work directly with each other, but here that is essential, and working very well, and perhaps is a model for the future of integrated cancer care and what may be possible with gene and cell therapy for curative cancer intent,« Dr. Lou explains and continues:
»The average approach to cancer treatment that a solid tumour oncologist is most familiar with may be quite different in bone marrow transplants. Bone marrow transplant standard operating procedures are quite different than what we do in solid tumours. So we have had to educate each other,« he adds, also mentioning that some meetings have included up to 50 people in one call.
Not a Miracle Cure – Yet
So far several patients have been enrolled, and the first results are expected to be reported in early 2021, which will add to the so far sparse data from CRISPR-edited therapy trials in cancer. No matter how promising the technology seems, it has yet to prove its worth.
»I am very careful when patients read about CRISPR and are excited about our trial and think it might be a miracle cure. I try to be very objective and transparent to make sure they understand the risks versus potential benefits. At the same time, I believe the trial strategy is based on sound science and I am optimistic and eager to see the outcomes from this treatment,« Dr. Lou states.
He hopes that within the coming years doctors will have a better body of data from this and other trials, and will able to say that not only it can be administered safely, but that it will also have been proven as a new frontier for treatment of patients with metastatic solid cancers.
»We do trials to find answers. This trial provides a very innovative strategy, and we are very hopeful that we will have much more of an impact than currently available therapies. But we are not in a position to promise anybody anything until we move forward and make our assessment objectively with RECIST criteria for Partial and Complete Response (PR/CR). I think the patients who are coming in are very hopeful, and I know they would be absolutely thrilled to be cured. And I would be thrilled to be the doctor who can offer them something that may provide some hope for cure or meaningful treatment efficacy to extend quality and duration of life at such an advanced stage of their disease,« Dr. Lou says.
Tags
ArticleInterviewGastro-Intestinal Cancer, GISolid TumoursCRISPR-CasCas9TrialsClinical
CLINICAL TRIALS
Sponsors:
Suzhou Maximum Bio-tech Co., Ltd.
Sponsors:
Zhejiang University







