Gene Replacement Emerges as New Immunodeficiency Therapy

"David, the bubble boy" lived most of his life in a sterile plastic chamber. He was born with severe combined immunodeficiency (SCID), which comprises a group of monogenic disorders characterised by defects in lymphocyte development that dramatically weaken the immune system. Like other patients with SCID, David was exceptionally susceptible to pathogens, and he ultimately died from complications due to an otherwise innocuous infection with Epstein-Barr virus at age 12.
SCID is traditionally treated with an allogeneic bone marrow transplant, and this concept suggested that the disease could be cured by correcting the patient's own haematopoietic stem cells. So, in the early 1990s, the history of gene therapy began with so-called gene addition, where a functional copy of the mutated, disease-causing gene was introduced by a viral vector.
Gene addition turned out to work well for some disease-related genes, and significant success was eventually seen in the treatment of SCID-X1 and SCID-ADA with gene therapy using lentiviral delivery of IL2RG or ADA, respectively.
»However, for genes like RAG1 and RAG2, regulation is crucial. RAG1 and RAG2 function similarly to CRISPR by cutting DNA during B-cell and T-cell development, a process called V(D)J recombination, which generates diversity in antibodies and T-cell receptors. These genes must be highly regulated to avoid genomic instability and transformation. Therefore, genome editing is preferable for diseases involving RAG1 and RAG2 compared to gene addition,« says Ayal Hendel, who is an associate professor at Bar-Ilan University in Israel.
Gene addition is insufficient for highly regulated genes
While his group focuses on RAG2, Italian scientists from San Raffaele-Telethon Institute for Gene Therapy (SR-Tiget) in Milan headed by Professor Luigi Naldini and Anna Villa – a researcher also at the Institute of Genetic and Biomedical Research (IRGB), CNR - focus on RAG1. The Israelian and Italian groups have independently published two recent papers in Nature Communications and Science Translational Medicine, respectively, demonstrating a gene-editing approach to replace the gene's coding sequence while retaining all regulatory sequences, such as promoters, introns, and untranslated regions (UTRs).
“Our idea was to cut the exon just after the start codon and replace the entire coding sequence”Ayal Hendel
Both RAG genes have only a single coding exon – exon 2 for RAG1 and exon 3 for RAG2 – and there are many different mutations in each of these genes across patients. Ayal Hendel explains:
»We wanted to develop one solution that fits all, using one guide RNA and one donor DNA to replace the broken gene. Our idea was to cut the exon just after the start codon and replace the entire coding sequence. The strategy involves using donor DNA with two arms of homology: one arm starts at the break on the left side, and the second arm starts at the end of the replacement sequence.«
Maria Carmina Castiello, PhD and staff scientist in Anna Villa's group, is the first author of their paper, which describes two approaches for gene editing of RAG1: a targeting strategy and a replacement strategy. She elaborates:
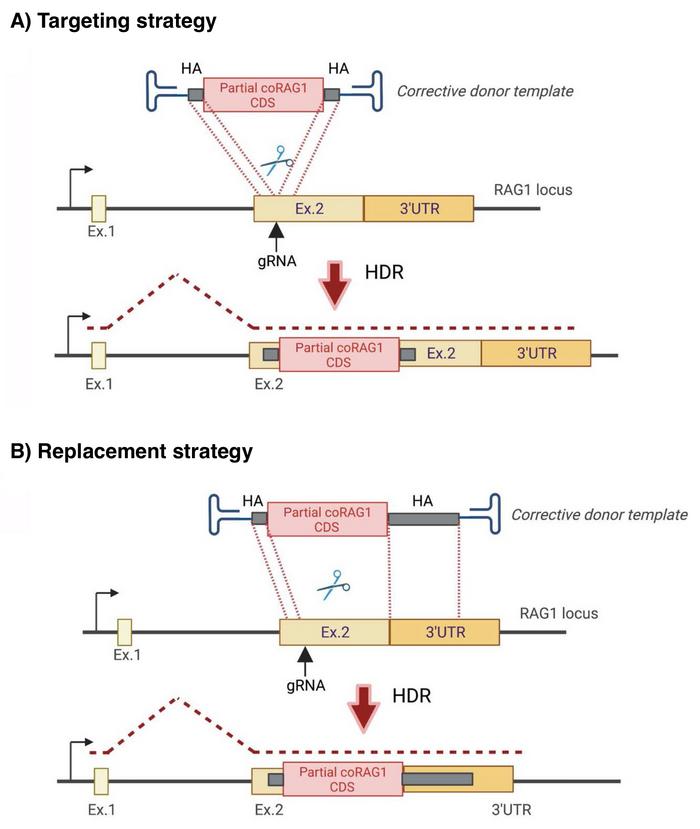
» In parallel with the targeting gene editing strategy into RAG1 exon 2, we developed a replacement template, including a long homology arm homologous to the 3' UTR, to allow the replacement of the endogenous gene with our corrected template. These approaches allow us to preserve the intronic regulation and rebuild the endogenous genomic conformation, so the untranslated region remains intact.«
These strategies ensure that all regulatory sequences are preserved and maintains the three-dimensional conformation of the gene locus, which is essential for the correct regulation of complex genes. This is particularly relevant for highly regulated genes like RAG1 and RAG2, where any disruption in their regulation could lead to immune dysregulation, genomic instability and transformation risks.
Gene replacement restores differentiation
The two research groups optimised their respective experimental strategies on haematopoietic stem and progenitor cells (HSPCs) from healthy donors and achieved on-target editing efficiencies with homology-directed repair (HDR) of up to around 30%.
After gene replacement, Ayal Hendel's team confirmed in in vitro assays that the edited HSPCs expressed the corrected RAG2 mRNA in a tightly controlled fashion. Moreover, they were able to differentiate into the expected T-cell lineages and to express a normal T-cell receptor (TCR) repertoire.
“We have to apply our methodology at a large scale and then conduct genotoxicity, off-target, and transgene integration studies to demonstrate safety”Ayal Hendel
Maria Carmina Castiello's group also tested their gene targeting and replacement strategies in patient-derived HSPCs by transplanting the edited cells into NSG mice lacking functional T and B lymphocytes. The edited cells demonstrated improved differentiation and development of both B and T lymphocytes in the transplanted mice, supporting the strategy's therapeutic potential.
»We are editing haematopoietic stem and progenitor cells, which, once edited, engraft into the bone marrow niche and are intended to repopulate the haematopoietic compartment for life, similar to stem cell transplants. Indeed, these edited autologous stem cells, with the permanent correction at the genomic level, retain their self-renewal and differentiation capacities,« says the Italian researcher.
Ayal Hendel highlights the importance of ensuring the safety of therapeutic RAG gene replacement. First, animal experiments must confirm that transplanted, engineered cells develop into healthy blood and immune cells without any signs of transformation or abnormal development. Secondly, comprehensive genotoxicity experiments must be conducted.
»We have to apply our methodology at a large scale and then conduct genotoxicity, off-target, and transgene integration studies to demonstrate safety,« he explains.
Progress offers new hope for SCID patients
Another concern for possible clinical translation is the method for delivering donor DNA templates. While Ayal Hendel's group uses recombinant adeno-associated virus (AAV), Maria Carmina Castiello and her colleagues also find that integrase-deficient lentiviral vectors (IDLV) are effective.
“The urgency of treating these patients as early as possible, ideally before 3.5 months of age, emphasises the potential of this therapy as an alternative to traditional transplants when a suitable donor isn’t available”Maria Carmina Castiello
This difference highlights an ongoing debate about the efficacy and safety of these vectors, with concerns about their toxicity to cells. The toxicity arises because cells recognise the introduced foreign DNA and activate a DNA damage response, which can harm the cells. AAV is exploited in many clinical programs, while IDLV has yet to be used in clinical trials for genome editing.
Both scientists acknowledge that gene editing strategies for RAG-dependent SCID are challenged by pricing. Maria Carmina Castiello explains:
»Since RAG-deficiency is a rare disease, the initial phases of clinical trials might not be profitable. The small number of patients makes it difficult for companies to invest without the promise of early returns. However, the urgency of treating these patients as early as possible, ideally before 3.5 months of age, emphasises the potential of this therapy as an alternative to traditional transplants when a suitable donor isn't available.«
The advances in gene editing for SCID, mainly through the precise replacement of RAG1 and RAG2 genes, mark a significant milestone in the quest to cure this debilitating disease. While challenges remain, the promising results from current research underscore the potential of these therapies to provide a lifelong solution for SCID patients.
As scientists continue to refine these techniques, the hope for a future where no child has to live in a sterile bubble becomes increasingly attainable.
Link to Ayal Hendel's original article in Nature Communications:
Link to Maria Carmina Castiello's original article in Science Translational Medicine:
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleInterviewNewsAdeno-associated virus (AAV)Lentivirus (LV)Severe Combined Immunodeficiency, SCIDImmunodeficiencies
CLINICAL TRIALS
Sponsors:
Suzhou Maximum Bio-tech Co., Ltd.
Sponsors:
Zhejiang University