Move over CRISPR: a new platform, TGEE, could soon replace everybody's favorite gene editing tool. Interview: Yoel Shiboleth, CEO TargetGene
By: Rasmus Kragh Jakobsen
(Interview is condensed and edited for clarity)

- So you are developing a system that can replace everybody's favorite gene-editing tool?
That is what we hope for. What we have designed is a nucleoprotein, which is programmed by different RNAs to target virtually any DNA sequence. Very similar in that respect to CRISPR.
- a protein like Cas?
Precisely the same idea: A protein programmed by a short RNA. Part of the RNA binds to the protein, and part of it binds to the target. The part that binds the protein is constant - an RNA loop that always binds the same domain on the protein. So we can change RNAs exactly like CRISPR.
But the big difference between us and CRISPR is the specificity. Our system is many orders of magnitude more precise.
- how is that?
Because it uses two RNAs instead of just one. Essentially our system is half of a dimer from a nuclease and an RNA binding domain. Then you have the same protein on each side but programmed with a different RNA on each side, and it cuts precisely in the middle.
- So two stretches of DNA that you target, and a fixed length of sequence in between?
Correct, it's a fixed angle because the helix turns. The two protein halves must reach each other. Our system is compulsorily dimeric - it cannot cut with a single protein.
In the cell, the two parts will self-assemble, and today we have a very efficient and very precise system working in human cells.
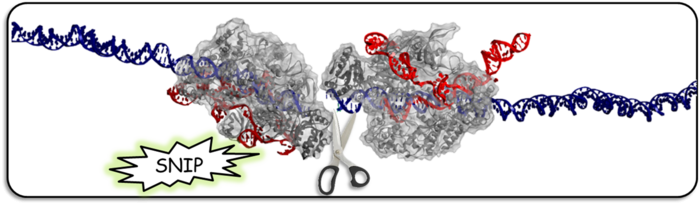
High Precision Genome Editing
Targetgene Biotechnologies has designed a unique protein they call TGEE (TargetGene Genome-Editing Engine). Unlike CRISPR, it is not found in nature, but like CRISPR, the basic idea is to break DNA in a specific place using DNA cutting enzymes. Also like CRISPR, the TGEE protein uses RNA-strings to guide the 'DNA scissors' to its target. However, TGEE has to identify two different DNA sequences separated by an exact number of nucleotides.
Two TGEE proteins, each with a different RNA guide bind the DNA, and only when the proteins form a dimer can it cut.
This double guide RNA requirement makes the system much more specific than CRISPR and unlikely to cut any unintended off-target sites in the genome.
Efficient and highly specific
- ok, how efficient is the system?
Currently, it's about half of CRISPR. But that is without many optimization, and we should be able to get as high as CRISPR.
You can improve efficiency on many different levels fx time and concentration. With CRISPR, the higher you go in concentration and the longer in time, the better the efficiency is, but also the more off-targets you get.
So it's a balance.
We don't have to abide by that balance because we do not have off-targets. There is no chance you will find a random sequence in the human genome that our guides will find, especially if you do a little bioinformatics.
- because of the requirement to have two target sequences separated by a defined angle?
Yes. For CRISPR, just 15 bases out of 20 are enough to target, but in the 6 billion base pair human genome that means you will get between 3 and 3.000 off-target events per each guide RNA. Even with algorithms to get rid of guides that have a lot of off-targets predicted, it is nearly impossible.
Now each extra nucleotide will add 4-fold more specificity, so in our system, using the same parameters, if we take two guides of 15 on one side and 15 on the other, with the specified distance between the two, there is absolutely no chance for off-targets.
- And specificity is, of course, key for going into treatments?
Absolutely. Questions of specificity are critical for therapeutics - if you are curing a chronic genetic disease, then you don't want those patients to risk having cancer or other problems afterwards.
You want to be very sure the risk of off-targets is acceptable, and we think the risk of CRISPR today is unacceptable for most therapeutic applications.
“we have specificity that is at least a million times better”
We believe we will replace CRISPR because we have a specificity that is at least a million times better and if we also have an efficiency that is high enough. I think any CRISPR application people develop we will be able to follow in their path with a better system.
- And are you able to show this?
Not yet. We want to show side by side CRISPR versus our system on a relevant gene, showing that CRISPR has more off-targets and thus more risk of unwanted problems. Systems to look for off-targets like GuideSEQ can only detect a sensitivity at 0,1% - and CRISPR gives an off-target very near the detection limit, and we get none, so you can't see how much better ours is.
Yes, we would be happy to have the sensitivity to find all the problems of CRISPR. We want to win by merit.
Therapeutics - cancer, heritable disease and autoimmune disease
- What therapeutic targets are you developing?
I can only discuss this in general terms, but we are targeting three. The first is cancer, and one obvious possibility that can be done with a precise system like ours is checkpoint inhibitors.
- right, like immunotherapy?
Exactly. Just for the example PD1, when you knock out PD1, then those cells will be immune to the PDL signal that tells the T-cell not to kill the tumor. If you knock out PD1, you increase the chance to kill PDL-expressing cancer cells.
The second is heriditary diseases. We are looking for simpler ones - heritable monogenic disorders of the blood or ones where you can deliver the system locally like the eye.
The third target is autoimmune diseases. Those where your body is attacking an antigen made by your own body, and if you remove the auto-antigen, you remove the cause of the illness.
We intend to develop at least one indication to the end ourselves.

Going to phase 1
- and what is your current status?
We are now looking for investors that believe in us personally and understand that this technology is a huge game-changer. And it doesn't need the big-spending because we are stepping in the footsteps of others.
We don't need to develop new delivery methods, and we don't need to convince the FDA that genome editing is ok, because the large CRISPR companies have already done it.
Many things have been developed that allow us to go much faster and thus much cheaper forward and get to the value of those large companies as quickly as possible.
- and the timeline for the best-case scenario?
I think we can get FDA approval in about a year and a half for the first application going forward to phase 1 clinical. We are now looking for something that will bring us past the animal studies and into the production of materials needed for phase 1 and for FDA approval.
That I think can be done in a year and a half. It is optimistic, of course, but we have beaten our own deadlines quite often.
- Ok, great thank You very much for talking to us.
"We are the first to invent RNA guided nuclease" - TargentGene Short History
The idea for TargetGene Biotechnologies was born in 2011 when Yoel Shiboleth and Dan Weinthal returned to Israel from their post-docs in the US. Discussing frustrations with zinc fingers, which has to be replanned and the proteins redesigned for each new target, they envisioned a programmable system using RNA to guide the nuclease to its DNA target. It sounds familiar today - just like CRISPR - but back in 2011, when the pair sent their first patent application, nobody had heard of CRISPR except as a way for bacteria to make themselves immune against phages in yogurt.
»We are the first to invent RNA guided nuclease,« claims Yoel Shiboleth.
In 2012 the CRISPR papers came as a complete surprise, but at the same time, the articles were beneficial. Weinthal and Shiboleth were looking for investors, but found it very hard because nobody thought it would work. The CRISPR paper was a proof of concept, and the first investor was an agrochemical company Makhteshim Agan now called Adama.
The original aim was to target genes in plants, but later Adama spun off the technology to Monsanto (later acquired by Bayer).
TargetGene is now focusing on therapeutics and has been developing very carefully under the radar until now.
Tags
ArticleInsightInterviewCancerGene therapyOff-targetQuality ControlTargetGene Biotechnologies
CLINICAL TRIALS
Sponsors:
Suzhou Maximum Bio-tech Co., Ltd.
Sponsors:
Zhejiang University







