nChroma Bio clears first clinical hurdle for CRISPR-based epigenetic HBV therapy
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
The decision moves one of the most advanced in vivo epigenetic editing programmes into the clinic, at a time when the HBV field is increasingly focused on finite-course, functional cure strategies rather than lifelong viral suppression. It also places CRISPR-derived transcriptional regulation, rather than DNA cutting, under clinical evaluation for a chronic viral disease
“This regulatory clearance marks a defining milestone for nChroma and for patients affected by chronic HBV”Jeff Walsh, CEO of nChroma Bio
The Certificate for Clinical Trial enables nChroma to begin human testing of CRMA-1001 in patients with chronic hepatitis B virus (HBV) infection, a condition affecting more than 250 million people globally, and for which current antiviral therapies rarely achieve a functional cure. The trial will be conducted in Hong Kong, with Professor Man-Fung Yuen of The University of Hong Kong serving as principal investigator.
»Hepatitis B remains a major global public health challenge that can lead to life-threatening conditions such as cirrhosis and liver cancer,« says Yuen. »As a novel epigenetic silencer, I am enthusiastic about the promising potential of CRMA-1001 to achieve a functional cure for persons living with HBV, and I look forward to contributing to the development effort that could change the future for millions of people worldwide.«
CRMA-1001 represents a departure from classical CRISPR gene-editing strategies that rely on DNA cleavage. Instead, the therapy uses a catalytically inactive Cas9 (dCas9) fused to proprietary epigenetic effector domains to durably silence viral gene expression without cutting or nicking DNA (see Figure 1).
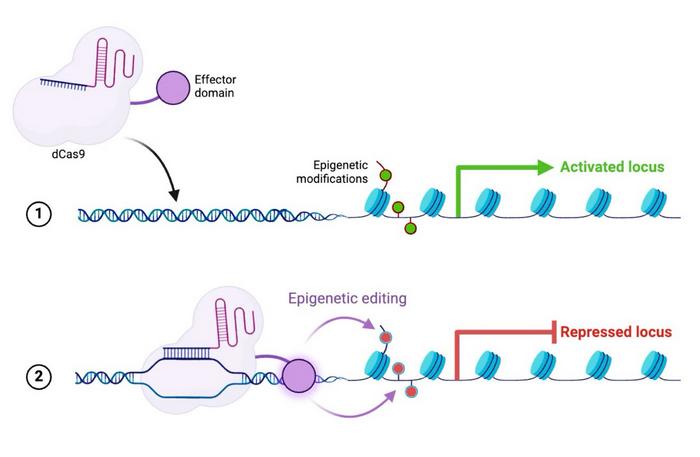
The approach targets both covalently closed circular DNA (cccDNA), the persistent episomal reservoir that underpins HBV chronicity, and integrated HBV DNA (intDNA) embedded in the host genome. By inducing targeted DNA methylation at these viral loci, CRMA-1001 aims to shut down antigen production at the transcriptional level, potentially enabling a finite-course, functional cure.
This distinction is not merely semantic. By avoiding double-strand breaks, epigenetic silencing may reduce risks associated with permanent genome modification, while offering durability that RNA-based approaches such as siRNA or antisense oligonucleotides often lack.
The regulatory clearance follows a series of preclinical datasets presented in November at The Liver Meeting 2025, where CRMA-1001 was highlighted in multiple abstracts, including a Poster of Distinction.
The approach targets both covalently closed circular DNA (cccDNA), the persistent episomal reservoir that underpins HBV chronicity, and integrated HBV DNA (intDNA) embedded in the host genome. By inducing targeted DNA methylation at these viral loci, CRMA-1001 aims to shut down antigen production at the transcriptional level, potentially enabling a finite-course, functional cure.
This distinction is not merely semantic. By avoiding double-strand breaks, epigenetic silencing may reduce risks associated with permanent genome modification, while offering durability that RNA-based approaches such as siRNA or antisense oligonucleotides often lack.
The regulatory clearance follows a series of preclinical datasets presented in November at The Liver Meeting 2025, where CRMA-1001 was highlighted in multiple abstracts, including a Poster of Distinction.
“As a novel epigenetic silencer, I am enthusiastic about the promising potential of CRMA-1001 to achieve a functional cure for persons living with HBV”Man-Fung Yuen, The University of Hong Kong
Across transgenic and AAV-HBV mouse models, CRMA-1001 achieved greater than three-log reductions in hepatitis B surface antigen (HBsAg), with durability extending to at least six months. At the highest dose, up to 90% of treated mice exhibited undetectable levels of both HBsAg and HBV DNA, suggesting deep suppression of viral transcription from both cccDNA and integrated sources.
Non-human primate studies, using PCSK9 as a surrogate liver target delivered via the same lipid nanoparticle system, further supported the platform’s durability and safety. A single dose produced sustained gene silencing for more than one year, with acceptable specificity, biodistribution, and safety profiles.
»These findings underscore the potential of CRMA-1001’s next-generation epigenetic silencing approach to achieve a durable functional cure for individuals living with chronic HBV,« the company stated when presenting the preclinical data.
The upcoming Phase 1/2 study will primarily assess safety and tolerability, as well as early signals of antiviral activity. While functional cure remains an aspirational endpoint, the mechanistic rationale has drawn attention from clinicians long frustrated by the limitations of lifelong viral suppression.
»This regulatory clearance marks a defining milestone for nChroma and for patients affected by chronic HBV,« says Jeff Walsh, CEO of nChroma Bio. »We see a real opportunity to change the trajectory of chronic hepatitis B by delivering deeper, more sustained responses driven by potentially best-in-class potency, going beyond the limits of existing and investigational treatments.«
If successful, CRMA-1001 would not only validate nChroma’s disease-first approach to genetic medicines but also strengthen the case for CRISPR-based epigenetic regulation as a therapeutic modality distinct from both traditional gene editing and transient RNA therapies.
For the broader CRISPR medicine landscape, the programme highlights a strategic pivot: from rewriting genomes to reprogramming gene expression – with durability, safety, and reversibility emerging as central design principles as the field moves deeper into the clinic.
The news was presented in a press release on 16 December 2025.
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleNewsClinical News UpdatesIn vivoHepatitis B virusLiver diseasesEpigenome editing (e-GE)dCas9Chroma MedicineNvelop TherapeuticsClinicalPre-clinical
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







