Organ-Specific In Vivo CRISPR With Cas12a Nanoclew Assemblies
CRISPR has taken the research world by storm and the first clinical trials results indicate that CRISPR is a safe and beneficial gene editing tool in humans. However, full therapeutic translation of CRISPR is hampered by a lack of efficient methods to deliver gene editing reagents safely to target organs and tissues.
Most of the in vivo therapeutic efforts rely on viral carriers to deliver CRISPR reagents to cells of the circulatory system, but efforts to develop non-viral organ-specific therapies are lagging behind.
New research led by Zhen Gu at University of California, Los Angeles has resulted in an innovative non-viral CRISPR delivery method that has the potential to specifically target any organ or tissue in the body.
I recently interviewed Dr. Zhen Gu and Dr. Wujin Sun, first author of the article which was published in Science Advances last month.
»CRISPR-Cas is a very powerful tool that can theoretically treat any genetic disease. However, simply incubating CRISPR reagents with tissues or cells is not enough because physiological barriers prevent the reagents from reaching the nucleus. We wanted to develop a system that could shuttle CRISPR reagents across multiple physiological barriers for therapeutic efficacy«, said Wujin Sun about the motivation behind the work.
Unleashing the Potential of Cas12a for CRISPR Therapy
Most clinical efforts today are centered on the Cas9 endonuclease, but Zhen Gu’s group chose Cas12a for their study. Cas12a is a powerful and programmable DNA-editing tool with diagnostic and therapeutic potential and its properties are distinct from Cas9.
Firstly, it is less prone to off-target editing than Cas9. Cas12a requires only a single CRISPR RNA (crRNA) for target recognition, which is shorter than the gRNA required by Cas9, and Cas12a also has a lower molecular weight, and these are attractive features since delivery is influenced by the size of the material that must enter the cell.
»Given these attractive properties of Cas12a and that it is relatively under-investigated in the therapeutic CRISPR field, we sought to unleash its potential for therapeutic applications«, said Wujin Sun.
Building a Bioresponsive CRISPR Assembly Layer-by Layer
In his former lab at University of North Carolina, Zhen Gu led the development of a novel prototype for in vitro delivery of Cas9 reagants based on a so-called DNA nanoclew. A DNA nanoclew is a spherical core of DNA onto which CRISPR reagents and other materials may be layered, that together allow the assembly to cross the cell barrier and reach the nucleus, all while maintaining cell viability.
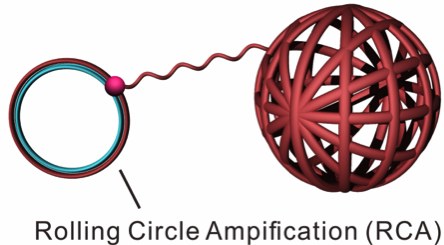
The nanoclew itself has a yarn-like appearance and it is synthesised enzymatically by rolling circle amplification – a rapid type of unidirectional nucleic acid replication that yields circles of nucleic acids (Figure 1). Because nanoclews are made of DNA, they are biocompatible and their ability to self-assemble makes them easy to customise.
In the latest work, the Gu lab advanced the nanoclew prototype for in vivo and tissue-specific delivery, but this time with Cas12a. First, Cas12a protein (from the Lachnospiraceae genus) was expressed in E. coli and then purified. It was then complexed with target-specific crRNA to generate a Cas12a/crRNA ribonucleoprotein (RNP) that constituted the gene editing cargo.
The group tested the complex’s in vitro cleavage activity using the enhanced green fluorescent protein gene (EGFP) as a target and they observed comparable cleavage in in vitro DNA cleavage assays and a model cell line, motivating them to proceed with Cas12a nanoclew development.
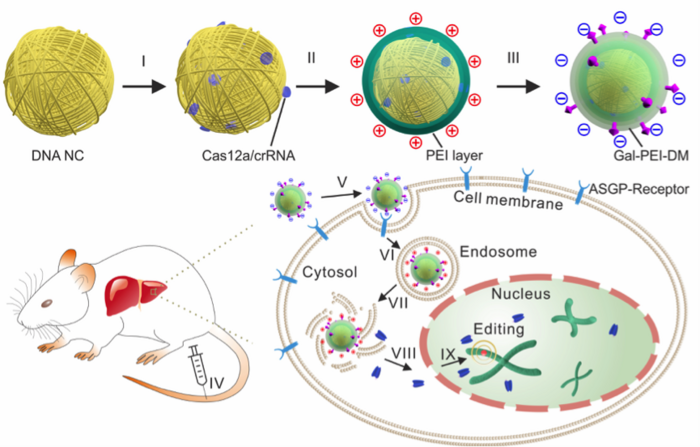
The group then synthesised nanoclews using a DNA template that complemented the target sequence, in this case either EGFP or another target gene, Pcsk9, which regulates serum cholesterol levels. The nanoclews were first loaded with the Cas12a-crRNA RNP, and then further layered until they could circumvent all the barriers for in vivo delivery. In vitro cellular assays, transmission electron microscopy and biochemical analysis allowed the group to identify efficient crRNAs as well as optimise conditions for loading, layering and trafficking of the nanoclews intracellularly (Figure 2).
Overcoming the Barriers to In Vivo CRISPR Delivery
»The ultimate goal of CRISPR-Cas12a delivery in our study was quite similar to the general goal of gene delivery. The cargo, in this case Cas12a and crRNA, must reach the nucleus of target cells in vivo. This goal is simple, but it is met with multiple barriers«, says Wujin Sun, and goes on to explain how the new assembly overcomes 4 major barriers.
1. The carrier needs to reach the target organ. Any systemically administered cargo can be easily cleared from the circulation and may distribute randomly in the body. Ensuring sufficient gene editing cargo in the target tissue is critical and depends on suitable formulation. The injected nanoclew assembly is therefore negatively charged which allows it to circulate for longer in the bloodstream.
2. The second barrier is the target cell membrane. Cargo that accumulates in the target organ but cannot enter the target cells will not work. The group modified the loaded nanoclew by conjugating a targeting ligand, in this case galactose, to help guide the assembly to the target tissue as well as to facilitate binding to the target cells i.e. hepatocytes.
3. Once the assembly enters target cells, the third barrier arises and this the endolysosome. These organelles tend to trap internalised cargos and may degrade them if they do not ‘escape’ in time. The addition of an anionic polymer layer to the nanoclew allows charge-reversal once inside the endolysosome. This means that once inside the organelle, the nanoclew becomes positively charged, which induces the so-called ‘proton sponge’ effect that disrupts the endosome membrane, allowing release of the nanoclew into the cytoplasm.
4. The ‘free’ CRISPR-Cas12a released from the nanoclew assembly now needs to bypass the final barrier by crossing by nuclear membrane. An engineered nuclear localisation signal on the Cas12a protein facilitates this step, bringing the assembly into the nucleus.
Successful in Vivo Target Editing with Cas12a Nanoclew Assembles
The PSCK9 protein is secreted by the liver and regulates cholesterol in the serum. Naturally occurring loss of function mutations in PSCK9 reduce the risk of cardiovascular diseases in some families and a number of PSCK9-inhibiting molecules have received FDA approval as cholesterol lowering agents. This makes Psck9 an potentially interesting target for gene therapy.
The group injected mice with Pcsk9-targeting Cas12a nanoclews and observed significant cargo accumulation in the liver as well as significantly (almost 90%) reduced serum PSCK9 levels and reduced cholesterol levels (45 % less serum than control). No hepatocyte toxicity was observed and no off-target effects were detected from the candidate off-target sites included in the study.
The Gu lab have previously shown the potential of targeting ligands to direct anticancer drugs to specific locations in the body. »In our lab, we have used targeting ligands to deliver anticancer therapies to different organs, such as brain and bone marrow. This is our first demonstration of using a targeting ligand to direct the delivery of CRISPR reagents. We believe our system has a broad potential by tuning physicochemical properties of our formulations as well as leveraging other targeting ligands toward different organs«, said Zhen Gu about the potential of the new assemblies.
Flexible, Smart, Biocompatible CRISPR Delivery
Most CRISPR delivery projects focus on developing Cas9 delivery systems. This new work reveals that Cas12a is at least as efficient as Cas9 in targeting therapeutically relevant genes in vivo. The group’s previous work indicates that nanoclew-based carriers can also be a platform for different CRISPR systems, e.g., Cas9. In fact, flexibility is a major aspect to the nanoclew platform.
»Our system is a ‘smart’ one that can change its surface charge depending on the physiological environment. It’s also composed of biocompatible materials that can be easily synthesised without the generation of environmental hazards. Its biodegradability reduces the concern for long-term nanomaterial accumulation in the body. Ultimately, we see our DNA-based carrier as a platform system, where the binding sequence can easily be adjusted to deliver different CRISPR tools«, said Zhen Gu.
»In the current study, we intravenously injected the assemblies to target the liver. Our platform overcomes both tissue level and cellular level barriers for efficient delivery. Besides systemic administration, nanoclews can also be used in local delivery systems. In that case, they will just need to overcome cellular level barriers. Without the loss during systemic circulation, it is possible that local delivery can lead to higher gene delivery efficacies«, adds Wujin Sun.
Future Plans
The risk of off-target editing is a major challenge for the development of CRISPR therapies in general. The currently study briefly addressed off-targeting of 3 potential sites that were selected based on similarity to the target site. Among the group’s future plans is a systemic investigation of the potential off-target effects of the Cas12a nanoclews. Commenting on the path to clinical development, Wujin Sun said: »There are many regulatory challenges to performing human trials but human-derived organoid models could be a good place for us to test our platform further.» Eventually, the group hopes to proceed with clinical development of the new platform. »But before that, we will further substantiate the efficacy and safety of our delivery vehicles with pre-clinical animal models«, said Zhen Gu.
Realising the potential of the RNA-targeting Cas13 system to treat viral infections, especially in light of the current COVID-19 pandemic, the group are also planning to design a Cas13 nanoclew delivery formulation with enhanced efficiency.
Link to original article in Science Advances:
CRISPR-Cas12a delivery by DNA-mediated bioresponsive editing for cholesterol regulation.
The Gu Lab
Dr. Zhen Gu’s research is focused on developing bioresponsive drug delivery systems that apply physiological signals-responsive materials and devices for enhanced controlled drug delivery. His group is devoted to inventing glucose-responsive synthetic formulations and devices for delivering insulin in a self-regulated manner. They have also leveraged physiological properties of different organs to engineer drug delivery systems that can target the diseased sites. Highlights of his group's inventions include “smart insulin patch”, platelet-delivered immunotherapy and DNA nanoclews for genome editing.
Tags
CLINICAL TRIALS
Sponsors:
Suzhou Maximum Bio-tech Co., Ltd.
Sponsors:
Zhejiang University







