Recent Clinical Updates From the Gene-Editing Field
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
Vor Bio presents positive data update on Trem-cel at ASTCT-EBMT 6th International Conference on Relapse After Transplant and Cellular Therapy
Earlier this month, Vor Bio announced positive clinical data from patients with acute myeloid leukaemia (AML) treated with trem-cel (VOR33) in the ongoing first-in-human Phase 1/2a VBP101 trial.
Trem-cel is Vor Bio’s lead engineered haematopoeitic stem cell (eHSC) product candidate and is developed from healthy donor haematopoietic stem and progenitor cells (HSPCs). These cells are CRISPR-Cas9-edited to delete the CD33 gene. CD33 is a cell surface antigen that is abundantly expressed in the vast majority of AML cases, and it is one of the main validated target antigens in AML to date.
Several programmes are ongoing to target CD33 in AML, but so far gemtuzumab ozogamicin (Mylotarg™) is the only CD33-directed antibody-drug conjugate to be approved by the FDA and European Medicines Agency (EMA) for the treatment of AML.
Transplant with trem-cel is designed to replace standard of care transplants for AML and potentially other blood cancer patients. The candidate is currently being investigated as a treatment for individuals with CD33+ AML at high risk of relapse after allogeneic haematopoietic cell transplantation (HCT), whereby trem-cel would allow post-HCT treatment with a CD33-targeting therapy without toxicity to engrafted cells.
The latest data, presented in an oral presentation at the ASTCT-EBMT 6th International Conference on Relapse After Transplant and Cellular Therapy (HSCT²), demonstrated primary neutrophil engraftment in all seven patients treated to date with trem-cel, with a median time to engraftment of 10 days. Platelet recovery at a median of 15.5 days was also demonstrated in six of these patients; anti-platelet antibodies were previously documented in the 7th patient.
Three patients enroled in the trial were treated with multiple cycles of Mylotarg, and all of these experienced haematologic protection from deep cytopenia (reduction in the numbers of several blood cell types), indicating that trem-cell protected the engrafted healthy cells from the on-target toxicity typically seen during Mylotarg treatment.
The company now plans to initiate dose escalation of Mylotarg to find the recommended Phase 2 dose.
Intellia shares new interim data from Phase 1 trial of NTLA-2001 in transthyretin amyloidosis
At the beginning of this month, Intellia Therapeutics shared additional interim data from the ongoing Phase 1 trial of NTLA-2001 in an oral presentation at the 4th International ATTR Amyloidosis Meeting, which was held in Madrid, Spain.
NTLA-2001 is jointly developed by Intellia Therapeutics and Regeneron and was the first in vivo CRISPR therapy to be administered to humans via the bloodstream. It is designed to be a single-dose curative treatment for transthyretin amyloidosis (ATTR, see Fact Box) by selectively reducing the levels of mutated TTR protein in the blood, through CRISPR-Cas9-based inactivation of the TTR gene in liver cells.
The Phase 1 trial is a two-part study evaluating NTLA-2001 in patients with either ATTR amyloidosis with cardiomyopathy (ATTR-CM) or hereditary ATTR amyloidosis with polyneuropathy (ATTRv-PN). In a press release, the company stated that the recent data, which had a cutoff date of May 11, 2023, are from the initial 65 out of 72 patients dosed in the Phase 1 study, which has now completed enrolment. The results from the final seven patients dosed, who were enrolled after the data cutoff, will be reported at a future date.
In summary, the new data revealed consistent, deep and durable reductions in serum TTR levels following a single dose of NTLA-2001, including in 29 patients who have now reached 12 months or more of follow-up. NTLA-2001 was generally well-tolerated across both polyneuropathy and cardiomyopathy arms at all dose levels tested.
The updated data for NTLA-2001 represents the largest clinical dataset for an in vivo CRISPR-based candidate therapy to date. Based on clinical data to date, the company has selected a 55 mg dose of NTLA-2001 for further evaluation in the global pivotal Phase 3 trial that has already received clearance by the FDA and is expected to begin by the end of 2023.
Transthyretin amyloidosis (ATTR)
Transthyretin amyloidosis (ATTR) is a rare, progressive disease, in which a protein known as TTR becomes misfolded and accumulates as plaques in tissues throughout the body. This causes serious complications that mainly involve the heart and nerves, and most patients die 2-15 years after disease onset. ATTR occurs in a heritable form (ATTRv) and an acquired form (ATTRwt) that may occur with elevated age (ATTRwt). The exact prevalance of ATTR is unknown but ATTRv amyloidosis is estimated to affect 50,000 people worldwide.
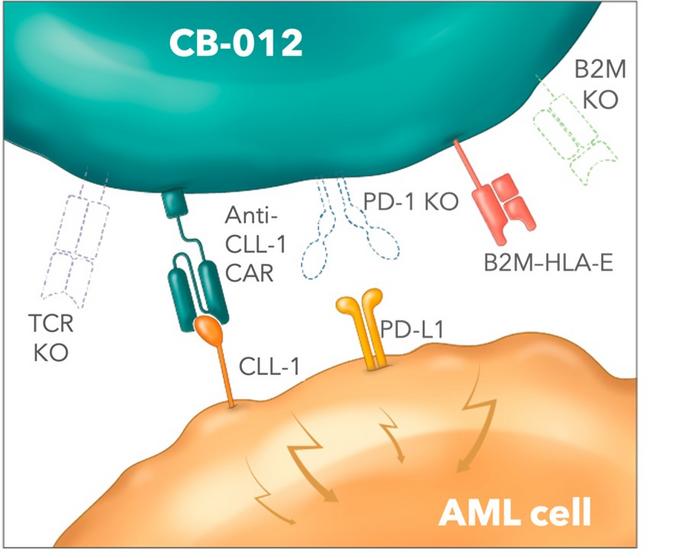
Caribou Biosciences gets FDA green light to proceed with novel CAR-T candidate for AML
Caribou Biosciences announced in October that the FDA had cleared its IND Application for CB-012, a novel donor-derived CAR-T cell therapy for the treatment of relapsed or refractory AML.
Specifically, CB-012 is an allogeneic anti-C-type lectin-like molecule-1 (anti-CLL-1) CAR-T cell therapeutic candidate that takes advantage of the abundant expression of CLL-1 on AML cells and leukaemic stem cells, while CLL-1 expression is absent in haematopoietic stem cells.
CB-012 contains five genomic edits, which are introduced using Caribou's patented CRISPR Cas12a chRDNA genome-editing technology. According to Caribou's press release, CB-012 is the first allogeneic CAR-T cell therapy to its knowledge with both checkpoint disruption through a PD-1 knockout, and immune cloaking through a B2M knockout and B2M–HLA-E fusion transgene insertion. Both of these armouring strategies have been designed to improve the anti-tumour activity of CB-012.
The company expects to initiate enrolment for the AMpLify Phase 1 clinical trial by mid-2024. This will be an open-label, multi-centre trial to evaluate CB-012 in adult patients with relapsed or refractory AML, and will comprise two parts. Part A, a 3+3 dose escalation design, will evaluate the safety and tolerability of CB-012 at increasing dose levels to determine the maximum tolerated dose and/or the recommended doses for expansion. Part B is the dose expansion portion with the primary objective of determining anti-tumour response, assessed by overall response rate (ORR), after a single dose of CB-012.
Learn more about Caribou's approach to therapeutic gene editing in our recent interview with CSO Steve Kanner PhD here.
We strive to bring you all the clinical updates for gene-edited therapies. For a complete overview of current gene-editing clinical therapeutic trials as well as diagnostic trials, check out CRISPR Medicine News' Clinical Trials Database.
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleNewsClinical News UpdatesAcute Myeloid Leukemia, AMLTransthyretin amyloidosis, ATTRCancerRare DiseaseCAR-TCRISPR-CasCaribou Biosciences, Inc.Intellia Therapeutics, Inc.Vor Biopharma
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







