Safety is Key to Novel CRISPR Medicines
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...

In October last year, we did an interview with Safety Director Roberto Nitsch atAstraZeneca, where he is focusing on the safety of potential future gene therapy initiatives. To most people, the safety of CRISPR is probably mainly a question about off-target editing, but Nitsch has a broader view:
»There are three key safety issues related to CRISPR Cas9 that the scientific community is studying right now. First off-target and on-target rearrangements, second intrinsic immunogenicity of Cas9, and third a p53 mediated response to double-strand breaks,« he says.
“We need to develop better technologies and address the safety issues”Roberto Nitsch
Nitsch also emphasises the safety concerns of both viral and non-viral delivery vectors. He believes, it is a common misconception that viral vectors are associated with more safety concerns than non-viral vectors, and he reminds that lipid nanoparticles also can generate an inflammatory response.
Read our full interview with Roberto Nitsch here.
Identification of off-targets leads to better guide RNAs
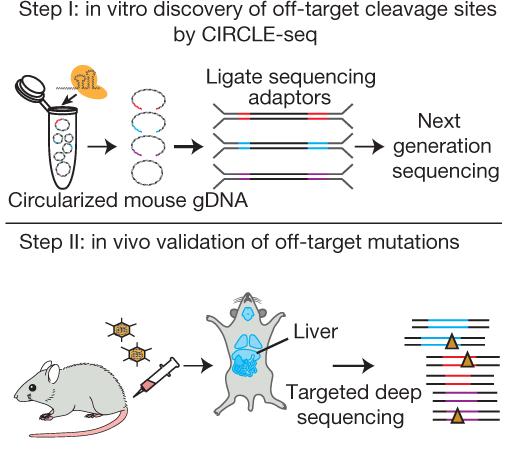
In February, we also talked to Marcello Maresca, who is Associate Director at AstraZeneca. Maresca is a corresponding author of a Nature paper from 2018 that describes a strategy to identify genome-wide off-target effects of CRISPR–Cas nucleases in vivo. The approach is called VIVO, and it is a useful tool in the design of guide RNAs.
»We find that with a well-designed guide targeting the PCSK9 gene, we tend to have no detectable off-target mutations in vivo,« Maresca explains. However, he also agrees with Roberto Nitsch that off-target editing is only part of the problems that need to be addressed with CRISPR medicines.
“When we can make a better target, we can make drugs that can better treat diseases”Marcello Maresca
Maresca believes that it is essential to identify and validate suitable targets when developing new drugs. And that is the main reason that AstraZeneca is using CRISPR.
»One of the reasons why many drug projects fail is not because we don't develop good drugs, but because we don't have good targets. So we tend now to use CRISPR to come up with good targets - that is, a target that, if modulated by a drug, will treat the pathological consequences of the disease,« he says.
Tags
ArticleNewsOff-targetQuality ControlCRISPR-CasCas9SafetyAstraZeneca
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.