Single-Dose Therapeutic Genome Editing Using Lipid Nanoparticles Shows Promise in Mouse Models of Rare Metabolic Disease
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...

Scientists at East China Normal University and YolTech Therapeutics in Shanghai developed a new treatment approach for primary hyperoxaluria type 1 (PH1), a rare but severe genetic disorder that can lead to kidney failure in young children.
In a proof-of-concept study, the team demonstrated that a single administration of CRISPR-Cas9 gene-editing technology using lipid nanoparticles (LNPs) disrupted the gene encoding glycolate oxidase, reduced urinary oxalate levels, and prevented kidney damage in mouse models of PH1.
The therapeutic effects after a single administration lasted for up to 12 months, with minimal off-target effects and no significant toxicity, offering a safer and more practical treatment approach than current therapies that require repeated dosing or organ transplantation.
»This study represents a significant step forward in developing a practical treatment for PH1,« said corresponding author and the founder and CEO of YolTech Therapeutics, Yuxuan Wu, PhD. »The ability to achieve long-lasting therapeutic effects with a single treatment could dramatically improve the lives of patients who currently require frequent medical interventions.«
The study was published in Molecular Therapy.
Need for more effective and safer therapies for PH1
PH1 is a life-threatening metabolic disorder caused by mutations in AGXT, which leads to dysfunction of the enzyme alanine-glyoxylate aminotransferase (AGT). AGT deficiency in the liver results in excessive production of oxalate, a metabolic byproduct that accumulates in the kidneys and forms calcium oxalate crystals. These crystals can cause kidney stones, kidney damage, and eventually kidney failure.
»There are relatively many PH1 patients in China who have to receive liver and kidney transplants. They are very young, many under 18 years old,« explained Dr. Wu. »These patients accumulate urinary oxalate, which creates crystals in the kidneys and causes renal damage.«
The disease typically manifests in early childhood, with a median age of 5.5 years. Dr. Emma Wang, chief technology officer at YolTech Therapeutics and study co-author, emphasised the limited treatment options currently available: »Other than liver transplantation, there is currently no other available treatment for PH1 in China.« She added that there are only two approved siRNA treatments globally, but these require regular dosing.
A novel therapeutic approach for PH1
The researchers sought to address the limitations of current PH1 management methods by developing a non-viral delivery system for CRISPR-Cas9 that could provide efficient, long-lasting therapeutic effects with a single administration and minimal side effects.
Their delivery approach focused on using lipid nanoparticles, which offer several advantages over viral vectors, including greater payload capacity, reduced immunogenicity, and the ability to deliver Cas9 mRNA transiently, thereby minimising off-target effects. This approach also offers easier and more cost-effective manufacturing.
The researchers designed and screened multiple sgRNAs targeting the mouse Hao1 gene, which encodes glycolate oxidase (GO), a key enzyme in oxalate production. They identified the most effective sgRNA and optimised the LNP formulation for efficient delivery to the liver while minimising off-target effects.
Dr. Wu explained the rationale for targeting HAO1 instead of attempting to correct the underlying AGXT mutation: »It’s very hard to correct AGXT mutations because every patient has different mutations. If you want to correct the AGXT mutations, you have to design different guide RNAs for different patients, making it a personalised treatment that would be very expensive.«
The authors also explained that the LNP delivery system offers several advantages over viral vectors.
»Lipid nanoparticles, by default, target the liver when administered intravenously. Since PH1 is a liver disease, LNPs are an ideal delivery vehicle,« explained Dr. Wang. She added that LNP-based therapy can be much cheaper than traditional viral-based delivery approaches and avoids the problem of preexisting immunity, which is found in roughly 80% of the population with AAV-based approaches.
Meet Emma Wang at CRISPRMED25!
★ Don't miss it: Emma Wang will present "In vivo genome editing: Translating science from bench to bedside" at the CRISPR Medicine Conference 2025 Virtual Event (April 7th online).
Register to attend CRISPRMED25 here!
In vivo validation of LNP-CRISPR-Cas9 gene-editing efficiency
The team created a PH1 mouse model to validate the gene-editing efficiency of their therapeutic approach in vivo. They used LNP-delivered CRISPR-Cas9 to target the mouse Agxt gene with an editing efficiency of 71.52% at a dose of 1 mg/kg. This approach resulted in a dose-dependent reduction in AGT expression, elevated urinary oxalate levels, and deposition of kidney calcium oxalate, thereby replicating the PH1 disease state.
The team then treated PH1 mice with LNP-CRISPR-Cas9 targeting the mouse Hao1 gene and assessed the treatment effects on gene-editing efficiency, GO expression, and urinary oxalate levels over short- and long-term periods (up to 12 months). The LNP-delivered CRISPR-Cas9 system achieved a high editing efficiency in the liver when LNP-CRISPR-Cas9-mHao1 was administered at the optimal dose. Editing efficiencies of 61.28% and 75.31% were achieved at doses of 0.3 mg/kg and 1 mg/kg, respectively (Figure 1). These editing efficiencies remained stable for up to 12 months after a single administration (72.62% at 6 months and 72.89% at 12 months), demonstrating the durability of the treatment.
Hao1 editing in the liver led to a significant reduction in GO mRNA and protein levels and, consequently, lower urinary oxalate levels in treated PH1 mice. Urinary oxalate levels were reduced by 59.19% and 64.71% in mice receiving 0.3 mg/kg and 1 mg/kg doses, respectively, compared to untreated PH1 controls. Moreover, LNP-CRISPR-Cas9-mHao1 treatment protected mice from nephrocalcinosis and kidney damage, even when challenged with prolonged ethylene glycol exposure, which typically worsens oxalate accumulation.
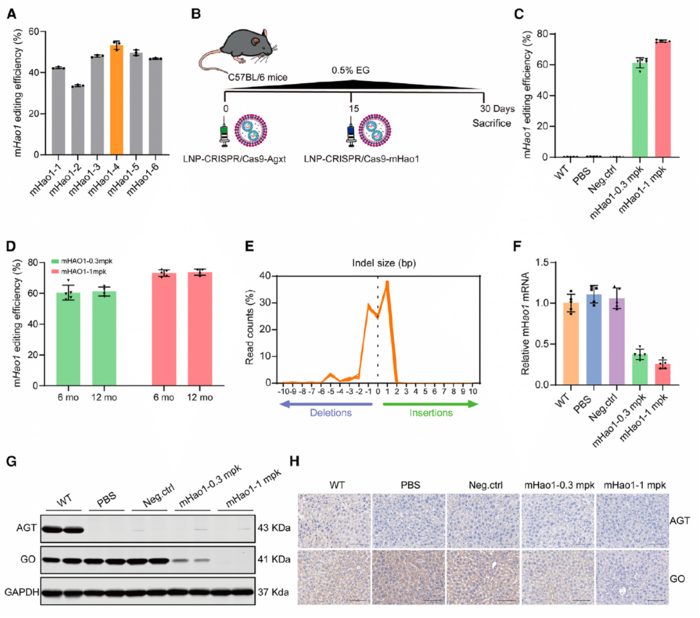
The therapeutic effects of LNP-CRISPR-Cas9 remained stable even after partial liver removal and regeneration, indicating that genetic modifications were maintained in new liver cells. According to Dr. Wu, this finding suggests that the treatment could provide lasting benefits, even as liver cells naturally turn over.
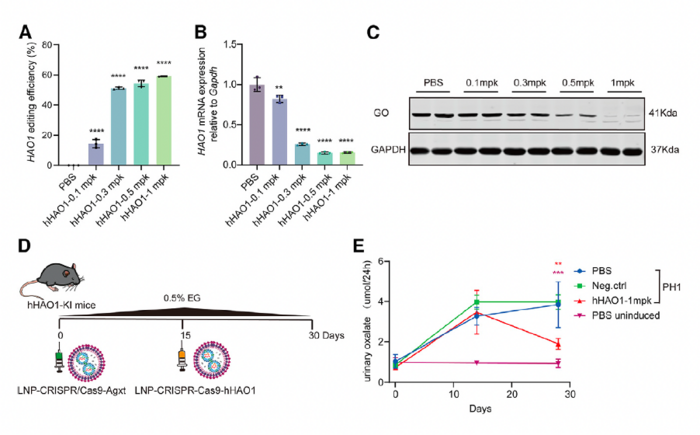
Validation of LNP-CRISPR-Cas9 efficacy in humanised PH1 mouse model
The researchers also generated a humanised HAO1 knock-in mouse model and tested the efficacy of LNP-CRISPR-Cas9 targeting the human HAO1 gene. This model allowed them to demonstrate that their approach could be effective with human gene sequences, which is an important step toward clinical translation.
In humanised mice, the therapy achieved editing efficiencies of up to 59.19% at the optimal dose of 1 mg/kg (Figure 2). In addition, treated mice showed reductions in urinary oxalate levels similar to those observed in PH1 mice. Notably, GO protein levels were almost undetectable at the 1 mg/kg dose. According to the authors, these findings in the humanised PH1 mouse model provided strong support for the clinical evaluation of this approach in human patients.
Safety and off-target effects
To evaluate the safety of their approach, the team analysed off-target effects, liver toxicity, inflammatory responses, and anti-Cas9 antibody production in mice administered LNP-CRISPR-Cas9. Treated mice showed no significant liver toxicity, with transient and mild liver enzyme elevations that normalised within 48 hours. No immune responses against Cas9 were detected, and histological examination showed no evidence of liver damage or inflammation.
Long-term follow-up revealed no signs of liver toxicity or other adverse effects for up to 12 months after treatment. Moreover, deep sequencing showed that the frequency of gene editing at off-target sites did not exceed the background indel rate, suggesting the specificity of CRISPR-Cas9 targeting.
First-in-human data
The researchers have already begun translating their in vivo findings to human patients. In an ongoing single-arm, open-label, Phase 1 trial, the team is evaluating the safety, tolerability, and efficacy of their single-dose therapeutic genome editing approach in Chinese children (2 Years and older) and adults with PH1.
At the time of this interview, Dr. Wang revealed preliminary unpublished results from seven patients who received 0.3–0.45 mg/kg of the LNP-CRISPR-Cas9 formulation, now known as YOLT-203.
»We see significant reduction in urinary oxalate, up to 75% in some patients who were dosed in our investigator-initiated trial,« Dr. Wang noted. »This is comparable to the efficacy of siRNA treatment, which requires quarterly administration.«
Dr. Wang also commented on the safety of the treatment in patients: »We saw no significant treatment-related serious adverse events or dose-limiting toxicities. As with other LNP-based therapies, we observe transient and mild liver enzyme elevation.« She further explained that liver enzyme levels returned to physiological levels within a day or two and that there have been no safety signals thus far. You can read more about the ongoing trial of YOLT-203 in a recent clinical trial update here.
The authors also revealed that in their clinical trial, they used a proprietary version of Cas12 instead of the Cas9 used in their pre-clinical studies.
»We have a discovery and evolution platform, where we mine metagenomic data to search for novel enzymes that are not patented by other companies,« explained Dr. Wang. »We have identified and optimised a Cas enzyme that is more efficacious than Cas9 for this disease target. We also performed off-target studies comparing Cas9 with our novel Cas enzyme, and found that our enzyme led to fewer off-target effects.«
Looking ahead
Based on the promising data from the mouse models and the investigator-initiated trial, the research team is moving forward with clinical development.
»This programme has received FDA orphan drug designation and rare paediatric disease designation, and we plan to submit an IND application to the FDA this year and potentially initiate a clinical trial in the US by the end of 2025,« Dr. Wang shared.
The team also emphasised their commitment to global accessibility and collaboration. »We want to find potential partners in the United States and Europe because we know there are many paediatric patients worldwide,” said Dr. Wu. »If we can collaborate with doctors and other companies worldwide, more patients can access better treatments.«
Link to the original article in Molecular Therapy:
Christos Evangelou, PhD, is a freelance medical writer and science communications consultant.
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleInterviewNewsPrimary hyperoxaluria (PH)YolTech Therapeutics
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







