The first CRISPR gene therapy is safe
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...

Setting a new milestone in CRISPR gene therapy, researchers have used the revolutionizing genome editing tool to edit the DNA of human immune cells to attack tumors in three patients. A man and two women in their 60's all had advanced refractory and metastatic cancer (one with sarcoma and two with the blood cancer multiple myeloma).
For these patients, the trial did not go as well as hoped.
One died in December, and the other two are in other therapies as the disease got worse.
»One patient had a tumor mass that decreased by more than 50%, and then it began to progress after four months,« study leader Carl June at the University of Pennsylvania writes in an email.
However, the study was designed to test the safety and feasibility, not cure cancer. No one knew how well CRISPR edited immune cells would be tolerated in patients, and by this measure, it's deemed a success.
»The study is an important milestone for the application of the technology. It provides safety data and reassurance that human treatments can be delivered after genome editing without obvious side effects‚« says professor Waseem Qasim, an expert of cell and gene therapy, University College London, who was not involved in the study.
And in a related Perspective in Science, Jennifer Hamilton and Jennifer Doudna both at the University of California, Berkeley, write, »these findings provide the cell engineering field with a guide for the safe production and non-immunogenic administration of gene-edited somatic cells.«
Three edits and one viral gene-transfer
Here is what was done in the study.
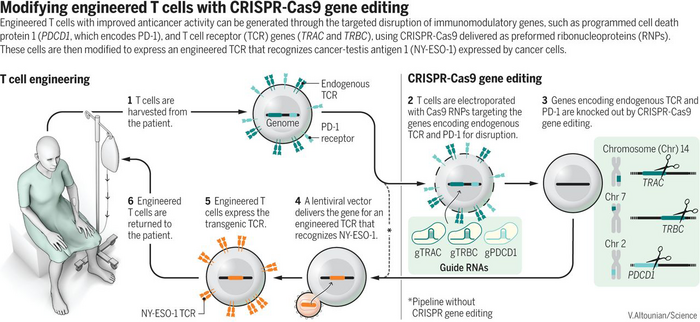
The trial builds on an earlier successful strategy that Carl June helped pioneer called CAR-T. By removing the patient's T cells and adding a gene for a receptor that recognizes a cancer antigen, then transferring the engineered cells back to the patient to attack the cancer cells. The CAR-T strategy recognizes cancer antigens on the surface of cancer cells, whereas the strategy of the new trial recognizes an intracellular cancer antigen represented on the surface of the cancer cells to the T cell receptor.
The endogenous antigen is called NY-ESO-1, and the researchers used a lentivirus vector to insert the gene for a T cell receptor that recognizes NY-ESO-1 in T cells harvested from the patients.
To further enhance the patient T cells, they first used CRISPR to disrupt three different genes. Two genes encoding the T cell receptors alpha and beta that might get in the way of the NY-ESO-1 T cell receptor were edited so that they were no longer expressed. The third gene is encoding the PD1 protein, which functions as a sort of safety switch or brake that cells can flip on to stop the T cells from destroying them. Many cancer cells exploit this to evade the immune response.
After 4-6 weeks, the T cells are transferred back to the patients who are intensely monitored to study the safety.
Just a baseline immune response
The patients tolerated the infused cells well, and the treatment was safe in that the researchers saw no toxicity such as cytokine release syndrome.
»The study confirms the preclinical data with CRISPR gene editing that it is flexible and it allows highly specific and multiple edits to be introduced into the human genome at one time,« says Carl June.
All the patients just had a baseline immune response to the Cas9, which June says was a welcome surprise since CRISPR-Cas9 is derived from bacteria that people previously have had infections with.
The trial also shows that immunogenicity did not present a barrier to the cells to be able to take root in the patients.
»The first patients now have had the cells survive in their blood for more than nine months, and the cells have retained their antitumor activity when we isolate them from the blood and test them in Petri dishes,« says Carl June.
So that is very encouraging. However, the outcomes for the patients were modest, which June suggests could be due to the target antigen that disappeared from the tumor, a process called 'tumor editing'.
»In one of the myeloma patients, the target antigen on the tumor cells was completely ablated, suggesting that the tumor cells had edited the tumor and that their persisting tumor cells no longer had the target. This will have to be addressed in future trials by combining multiple targets in the infused cells,« says Carl June.
Only rare off-target events
Besides the risk of triggering an immune reaction, the other big concern is for unintended off-target editing that could potentially turn the T cells cancerous. Not least because the genomes were cut in three different sites, but also here the trial data seems encouraging.
The scientists saw no signs of cancer and only found rare off-target events in the T cells from the CRISPR editing - according to June only about 10 off-target events out of more than 7,800 edits.
»The main safety concern that we found in our trial was that translocations could be observed during the manufacturing process and in the patients. The frequency of translocations decreased in the patients after infusion, suggesting that these translocations were not dangerous,« says Carl June.
In the new trial with the CRISPR edited cells frequency of translocations in the infused cells was less than 1%. Compared to a previous gene therapy trial to treat cancer conducted at the Great Ormond Street Hospital in London led by professor Waseem Qasim using a TALENS editing technology, where they measured translocations in 4% of the infused cells.
The technology has improved rapidly
The researchers point out that the technology has advanced rapidly since the trial began in 2016, and even though the trial originally planned to recruit 18 patients, it will not continue, because the reagents available today can make the genome editing much more specific. One recent advance is base editors that June says should be able to make so-called scarless editing possible, where there are no DNA breaks and, therefore, no chance of creating translocations in the genome.
CAR-T and CRISPR editing trial next
For the next trial, June's team will begin to use the CRISPR technology in CAR-T cells, which have one big advantage over the recombinant T cell receptors used in the pioneering CRISPR trial. The CAR does not need to be matched to the patient's HLA type - meaning it can be used to generate 'universal' T cells. This is what the researchers aim for.
»We can manufacture T cells from healthy donors, then can give them to cancer patients who themselves have very few healthy T cells that could be turned into CAR-T cells,« says Carl June.
Good start
Even though the first CRISPR cancer trial had limited benefits for the patients, it has passed the first big hurdle and shown that CRISPR edited cells can be transplanted safely and that the engineered cells don't wither away but stays on for a long time retaining their cancer-fighting ability.
»The big question that remains unanswered by this study is whether gene-edited, engineered T cells are effective against advanced cancer,« Doudna and Hamilton write in their perspective.
Still, CRISPR gene therapy seems off to a good start.
»We will need more experience to fully assess the promise of CRISPR technology, but at this time, it looks to be living up to the hype that has surrounded the CRISPR technology,« says Carl June.
Tags
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







