Trial Update: CRISPR-Edited Therapies for Sickle Cell Disease and Blood Cancer
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
This week’s clinical update looks at 3 clinical trials for the treatment of sickle cell disease (SCD), relapsed or refractory CD5-expressing T cell cancers and certain CD7-expressing T cell cancers. All therapies investigated in these trials are developed using CRISPR-Cas9 gene-editing technology.
CRISPR_SCD001 – A CRISPR-Edited Red Blood Cell Therapy For Severe Sickle Cell Disease
The first trial is an open-label, non-randomised, two-center, Phase 1/2 study of a sickle allele-modified haematopoeitic stem cell (HSPC) therapy in participants with severe SCD.
The trial sponsor is Mark Walters, MD at University of California, San Francisco Benioff Children's Hospital Oakland, in collaboration with University of California, Los Angeles and University of California, Berkeley.
During the study, 9 participants between the ages of 12 and 35 years old will receive a single intravenous transfusion of CRISPR_SCD001, which is essentially red blood cells that are CRISPR-edited to directly correct the mutation in the beta-globin gene responsible for sickle cell disease. This marks the first trial for a CRISPR therapy that directly repairs the genetic defect underlying SCD, in contrast to other ongoing trials that aim to compensate for the lack of adult haemoglobin by reactivating foetal haemoglobin.
Before transfusion, each partipicant will undergo a myeloablative conditioning procedure with the chemotherapeutic busulfan. Myeloablative conditioning destroys the cells in the bone marrow to prevent rejection of the transfused CRISPR_SCD001 therapy.
The estimated study start date i.e. the date on which the first participant is expected to be enrolled, is May 28th, 2021 and the study completion date i.e. the date whereby data is available on the last study participant is set to May 28th, 2026.
The primary endpoint of this trial will determine the safety of CRISPR_SCD001 through a 3+3 design with staggered enrollment and a pause in enrollment for safety review after each of the first 3 patients is dosed. After safety is assessed in the 3rd patient, enrollment of the next 3 patients will not be staggered. The first 6 subjects will be adults. If CRISPR_SCD001 is determined to be safe in these participants, the trial will continue to enroll 3 adolescents 12 - 18 years of age to evaluate safety in younger patients. The younger age cohort also will follow staggered enrollment.
Secondary endpoints involve determination of blood counts, haemoglobin status, assessment of disease symptoms as well as occurrence of disease-associated complications such as viral or fungal diseases. Secondary outcomes will be assessed according to disease-specific criteria at various stages during the first 24 months post-treatment.
CT125A – A CRISPR-Edited CAR T Therapy for Advanced Blood Cancers
The second trial is a single-center, single-arm exploratory study to evaluate the safety and efficacy of CT125A, which is a patient-derived anti-CD5 chimeric antigen receptor (CAR) T cell therapy for the treatment of relapsed or refractory CD5+ blood cancers.
The trial is sponsored by Huazhong University of Science and Technology in collaboration with Shanghai IASO Biotechnology, both in China.
The trial, which is listed as early Phase 1, will enroll 18 participants between the ages of 18 and 70 years old that have any of the following CD5+ relapsed or refractory blood cancers: chronic lymphocytic leukaemia (CLL), mantle cell lymphoma (MCL), diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), and peripheral T-cell lymphoma (PTCL).
While CAR T therapies have shown great promise for the treatment of B cell cancers, T cell cancers are more challenging, partially because of shared expression of most targetable surface markers between normal and cancerous T cells. In recent years the therapeutic potential of anti-CD5 CAR T cells to treat T cell cancers has attracted attention. T cell cancers express CD abundantly, while in healthy tissue, CD5 expression is mostly restricted to certain subclasses of T cells.
To generate CT125A, trial partipicants will undergo leukapheresis to isolate peripheral blood mononuclear cells from which CT125A cells are manufactured. The cells are transduced with a CD5-targting CAR, and then further developed by knocking out the endogenous CD5 using CRISPR-Cas9 technology to prevent fratricide (self-killing) during CAR T cell upscaling and manufacture.
After lymphodepletion, participants will receive a single IV dose of CT125A cells. The tolerability and safety of CT125A cells will be assessed according to the "3+3" dose escalation design as described above.
Primary outcome measures will evaluate safety and toxicity while secondary outcome measures involve evaluation of partial and overall response rats at various intervals up to 2 years post-treatment and in accordance with disease-specific parameters.
The estimated study start date is March 2021 and study completion is expected in December 2023.
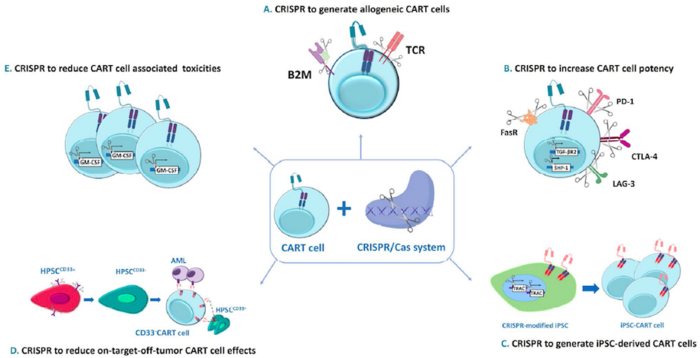
GC027 – A CRISPR-Edited CAR T Therapy for CD7+ T Cell Leukaemia and T Cell Lymphoma
The last trial in this week’s update is sponsored by Xinqiao Hospital of Chongqing China in collaboration with a number of industrial and hospital-based partners in China.
This trial is an early Phase 1 study for GC027, an anti-CD7 universal CAR T therapy for CD7+ T cell leukaemia and T cell lymphoma. The trial will take place in multiple centers in China and aims to enroll 30 participants between the ages or 2 and 70 years old. GC027 is developed by Gracell Biotechnologies in Shanghai using the company’s proprietary TruUCAR technology.
CD7 is a cell surface marker that is expressed on the surfaces of most cancerous T cells. To develop GC027, T cells are isolated from healthy donors, transduced with a CD7-targting CAR and gene-edited using CRISPR-Cas9 technology to avoid rejection following infusion into patients. The endogenous T cell receptor is also disrupted using CRISPR-Cas9 technology to mitigate the risk of graft-vs-host disease. Furthermore, the native CD7 locus is disrupted in GC027 cells to prevent fratricide during or after the manufacturing process. In this way, GC027 is being developed an off-the-shelf universal CAR T (UCAR-T) therapy that can be given to eligible patients without the need for immunosuppressive conditioning and without the need for donor human leukocyte antigen (HLA) matching.
Trial participants receive a single infusion of GC027 at one of 3 doses alongside standard chemotherapeutic treatments for their cancer. The primary study outcome is evaluation of the anti-tumor efficiency of GC027 at 4 weeks post-treatment and the secondary outcome measure is the long-term therapeutic efficiency at 3 and 6 months post-treatment.
Initial trial results presented at last year’s Virtual Meeting of the American Association for Cancer Research showed encouraging early response rates and manageable safety albeit toxicity that concerned some experts.
The trial is ongoing and is expected to be complete in April 2022.
For a complete overview of current gene editing clinical trials, check out CRISPR Medicine News' Clinical Trials Database.
Tags
ArticleNewsin vivoSickle Cell Disease, SCDT-Cell LymphomaCAR-TGracell Biotechnologies Co., LtdUniversity of CaliforniaCRISPR-CasCas9Huazhong University of Science and TechnologyXinqiao Hospital of ChongqingTrialsClinical
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







