Tuneable, Biodegradable, Lipid-Like Nanoparticles for In Vivo mRNA Delivery. Interview: Yizhou Dong, Ohio State University.
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...

Yizhou Dong PhD leads a research group at Ohio State University that is devoted to drug discovery and delivery for the treatment of genetic disorders, infectious diseases, and cancers. The Dong Lab is particularly interested in developing highly effective translational methods to deliver mRNA-based therapeutics and base editing technology for novel therapeutic medicines (see Fact Box).
Viral vectors are the most widely-used delivery vehicle for therapeutic genes, Cas endonucleases, guide RNAs and base editors, but their widespread applicability and safety is hampered by packaging limits i.e. the size of the material that can be packaged into a viral vector, as well as safety concerns.
Non-viral delivery strategies must account for the inherent vulnerability of mRNA molecules to enzymatic degradation by ribonucleases in biological systems, as well as their negative charge, which compromises their transit through cell membranes.
In recent years, a number of non-viral delivery vehicles have emerged. These include biocompatible carriers for mRNA such as lipid and polymer-based nanoparticles. Of these, the lipid nanoparticles have been extensively developed towards improved efficiency and low toxicity, but their biodegradability remains a concern.
The Dong Lab recently developed a series of biodegradable lipid-like nanoparticles that mediate efficient delivery of large therapeutically relevant mRNA molecules and base editing mRNAs in vivo. We interviewed Yizhou Dong to learn more about the work, which was recently published in Science Advances.
New, Biodegradable, Lipid-Like Nanoparticles
- Can you describe the new nanoparticles?
We previously developed a series of lipid-like compounds called TT3s and demonstrated that these could deliver human factor IX mRNA for haemophilia B therapy and induce gene editing on PCSK9 in different animal models. We published these results a few years ago.
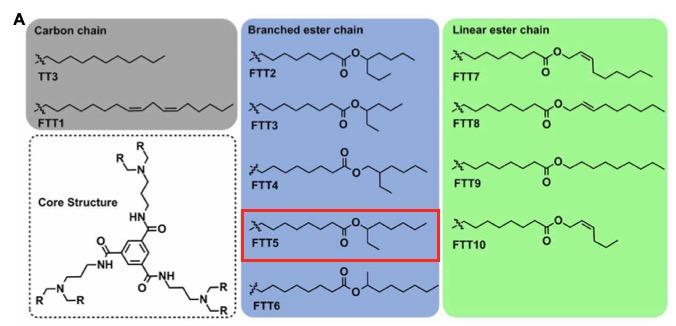
In our new study, we developed TT3 derivatives by combining their core structure with different biodegradable lipid chains. We then formulated these derivatives, which we call FTTs, into the new FTT lipid-like nanoparticles (FTT LLNs).
The FTTs fall into 3 categories, depending on the structure of their lipid chains, which are either comprised of carbon chains, branched esters or linear esters. Under an electron microscope, FTT LLNs appear quite homogeneous with a spherical structure. Their overall size, surface charge and morphology are quite similar to lipid nanoparticles in general.
- How did you test the new nanoparticles in vitro?
Once we had made the FTT LLNs, we tested their ability to enter a hepatocellular carcinoma cell line. We encapsulated firefly luciferase (FLuc) mRNA into the nanoparticles so that we could track their fate within the cells, and by measuring the resulting luminescence we found that they were just as efficient as the TT3s in delivering FLuc mRNA to cells.
We then moved to in vivo experiments where we injected various FFTs of different sizes and lipid chains intravenously and measured bioluminescence from the heart, liver, spleen, lung and kidney 6 hours after administration. Here we could see a clear preference for the liver among 10 different FTTs tested, and FTT5 showed higher mRNA delivery efficiency in the liver than TT3. Hence, we decided to proceed with FTT5 for subsequent experiments.
We also noticed that while the FTTs were rapidly cleared from the blood, they persisted somewhat in the liver and we could infer from the different FTTs tested that we could tune their biodegradability by modulating their lipid chains.
- And how do the FTTs bring nucleic acids into cells?
To help us understand how the FTTs are taken up by cells, we performed endocytic pathway studies, and we found that multiple pathways are involved in cellular uptake. Cells uptake different particles through various receptors, and each type of particle generally uses a unique cellular trafficking pathway to gain entry.
We performed inhibition studies using chemical inhibitors to selectively block the clatharin-mediated pathway, clatharin-independent pathway or macropinocytosis, and we found that all three pathways contributed to cellular uptake of FTT5 LLNs.
Additionally, FTT5 LLNs were able to rupture endosome membranes, releasing mRNA to the cell cytosol for the protein translation.
In Vivo mRNA Delivery
- You also showed that FFT5 nanoparticles could deliver human factor VIII mRNA and base editing machinery into mice. Can you tell us more about these experiments?
For the mRNA study, we injected FTT5 LLNs encapsulated with human factor VIII (hFVIII) mRNA into wild-type mice or FVIII knockout mice. FVIII is the clotting factor that is deficient in haemophilia A.
We measured circulating hFVIII in treated wild-type and haemophilic mice 6 and 12 hours after injection and found the levels to be within normal clinical range in all cases, without signs of pathological damage in the treated mice. Restoration of normal FVIII levels is a very important therapeutic parameter, and we also found the FVIII protein activity to be at 90% compared to normal levels.
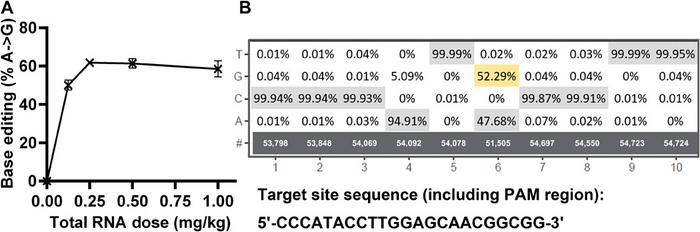
For the base-editing study, we targeted the PCSK9 protein. Many groups have targeted PCSK9 with CRISPR and other gene-editing strategies so there is much data available for reference. PCSK9 is also a relevant drug target, since it regulates blood cholesterol levels and its inhibition can treat certain types of cardiovascular diseases. We used the FTT5 LLNs to deliver mRNA encoding an adenine base editor and a single guide RNA targeting PCSK9 upon intravenous injection into mice.
We found that the FTT nanoparticles mediated base editing a rate of approx. 60 % at doses as low as 0.25 mg/kg of total RNA. To the best of our knowledge, this makes the FFT-delivered base-editing cargo the most potent in vivo base editor reported so far. We did the base editing work in collaboration with Beam Therapeutics and we will continue to collaborate to advance this application of our lipid-like nanoparticles.
- Do you foresee organ-specific delivery with the FTT5s?
In my lab, we work with many different types of materials and we usually try to match the therapeutic application with certain materials. Right now, for these new FTTs, we are mostly interested in pursuing therapeutic applications for the liver.
We have other ongoing projects for cancer immunotherapy with other types of nanoparticles that we can tune to different target organs, such as the spleen or tumour microenvironment.
In general, we have worked out many of the design principles for the chemical structures of the nanoparticles we use, such as which amino groups and lipid chains to include, particle size and physiochemical properties but it is still difficult right now to design these in a rational way so that they can overcome all the barriers we talked about i.e. enter specific target organs and release gene editing or mRNA cargo to the cytoplasm with high efficiency.
The New Nanoparticles Have Broad Therapeutic Potential
- Do you see other therapeutic uses for the lipid-like nanoparticles your lab is developing besides delivery of genomic therapies?
Yes, we see potential in the vaccine area. We recently published a study where we used TT3 nanoparticles to deliver SARS-CoV-2 mRNA sequences with optimised 5’ and 3’ untranslated regions into mice.
Vaccination with the TT3-delivered mRNA led to high expression of SARS-CoV-2 antigens and in vivo production of large amounts of specific antibodies against the Spike protein, which is the antigen most widely used in COVID-19 vaccine development efforts.
- What are the next steps?
We are working on many different projects. One is to explore the packaging capacity for the FTT nanoparticles. The FVIII and base-editing mRNAs we used are approx. 4.5 and 5.5 kb in length, respectively. In terms of packaging capacity these are pretty long molecules, and for the base editing experiments, we could also encapsulate the guide RNA into the same nanoparticle as the mRNA. So that is all very encouraging.
We haven’t tried to package a donor template for genome editing into our nanoparticles yet but we are working on this. In collaboration with Denise Sabatino at the Children’s Hospital of Philadelphia, we will try to insert a functional factor VIII gene into the mouse genome.
Another project is looking at oral delivery of nanoparticles. This is very challenging but it will have a high impact as it would be much easier for patients to take than injectable medication, either intravenously or otherwise.
Besides working on individual projects, we also continue to pursue our ultimate goal. That is to advance our lead materials and therapeutic cargos to clinical trials. We aim to do this by matching the material with the relevant therapeutic application and working with our university technology office and industrial collaborators in the hope that promising results will eventually lead to new approvals for clinical use.
Link to the original article in Science Advances:
Functionalized lipid-like nanoparticles for in vivo mRNA delivery and base editing.
(Interview is condensed and edited for clarity).
FACT BOX: mRNA-Based Therapeutics and Base Editing
mRNA-based therapeutics have the potential to treat genetic disorders by replacing missing or dysfunctional proteins, e.g., blood clotting factors, enzymes, and antibodies, without making permanent changes to the genome. Here, in vitro transcribed mRNAs are delivered to the body, where they then enlist the host’s protein synthesis machinery to undergo translation to the desired therapeutic proteins.
Base editing is a variation of CRISPR-Cas that uses an engineered Cas9 protein or mRNA encoding the engineered Cas9, which is guided by a single guide RNA to introduce specific point mutations at target site(s) in the genome. Base editing is an emerging technology that is gaining traction for the treatment of genetic disorders based on point mutations.
Since the Cas9 base editors are able to perform desired single base edits without making double-stranded breaks in the genomic DNA, base editing may be more attractive than conventional CRISPR-Cas editing from a safety point of view.
FACT BOX: Dong Lab
The research in Yizhou Dong's Lab at Ohio State University is devoted to drug discovery and delivery for the treatment of genetic disorders, infectious diseases, and cancers. Together with collaborators, the lab integrates its expertise in pharmaceutics, pharmaceutical chemistry, biomedical engineering, materials formulation, and animal studies to design novel therapeutic medicines and uncover their mechanisms of actions. The lab's ultimate goal is to translate its scientific results and technological inventions into more effective treatments and more fulfilling lives for all individuals.
Tags
ArticleInterviewDeliveryNon-viralLipid-based nanoparticleCOVID-19HaemophiliaGene therapyDivision of Pharmaceutics and Pharmaceutical Chemistry, Ohio State UniversityBase editors
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







