A Dual CRISPR Strategy Eliminates HIV in Humanised Mice

Professors Howard Gendelman and Kamel Khalili are men with a mission. They are determined to develop a one-shot cure for HIV-1 and use CRISPR to attack the virus on multiple fronts. An ongoing clinical trial aims to excise integrated proviral DNA in HIV patients. This strategy is now expanded in their laboratories in experiments to delete the CCR5 viral receptor for even better results in HIV-infected, humanised mice. And soon, an extra weapon – MOGS inactivation - will be deployed on top of that.
Howard Gendelman was a medical doctor in the Bronx in 1981, and here he saw some of the first HIV patients. The experience led him to a career at the University of Nebraska Medical Center, studying virus-cell interactions, viral pathogenicity, humanised mice as a model system, and the development of long-acting slow-effective release antiviral therapy (LASER ART).
“I looked at the integrated HIV genome as a cancer gene”Kamel Khalili
At the same time, Kamel Khalili, a molecular virologist from the Lewis Katz School of Medicine at Temple University in Philadelphia, pioneered a genetic approach to eliminating HIV. Already in 2014, he published his first attempt to use CRISPR for that purpose.
»I looked at the integrated HIV genome as a cancer gene. I thought that if I could eliminate the proviral DNA from the host genome, then we shouldn't worry about any medication because there won't be any virus to inactivate,« Kamel Khalili recalls.
HIV must be suppressed before CRISPR
But Kamel Khalili and Howard Gendelman needed each other to move on in their quest to eliminate HIV. LASER ART properties are defined by slow drug dissolution and maintain effective antiretroviral drug concentrations from days to weeks. While the therapy can keep HIV replication at almost undetectable levels, the virus still replicates and spreads to new cells. Low-level replication was also a challenge to the CRISPR strategy, as was the need to find a suitable model system.
»The merger of our two groups settled on two concepts. The first concept is to limit the number of targets seen by the CRISPR gRNAs by maximal virus suppression. So, we were developing targeted antiretroviral therapies to extend viral suppression and make the virus more amenable for targeting,« explains Howard Gendelman.
»Secondly, we needed a large cadre of humanised mice for all the experiments and controls associated with targeting, elimination, suppression, and ultimately eradication of the virus, and that wasn't easily accomplished by any other means.«
So, everything came together with Howard Gendelman's expertise in antiretroviral therapy (ART) and humanised mice and Kamel Khalili's knowledge of CRISPR. In their first series of experiments, they used CRISPR with two gRNAs, each targeting a site in the integrated viral genome. The strategy led to partial excision of the viral genome and abolished the production of infectious virus particles in several cell and small animal models. In a joint paper in 2019, the two researchers showed proof of concept that permanent viral elimination is possible in an HIV-infected humanised mouse model.
The Berlin patient showed the way
“We have a narrow window of less than two weeks to eliminate CCR5 before excising HIV from already-infected cells. So, the timing of CCR5 elimination and HIV excision had to be almost surgically manipulated”Howard Gendelman
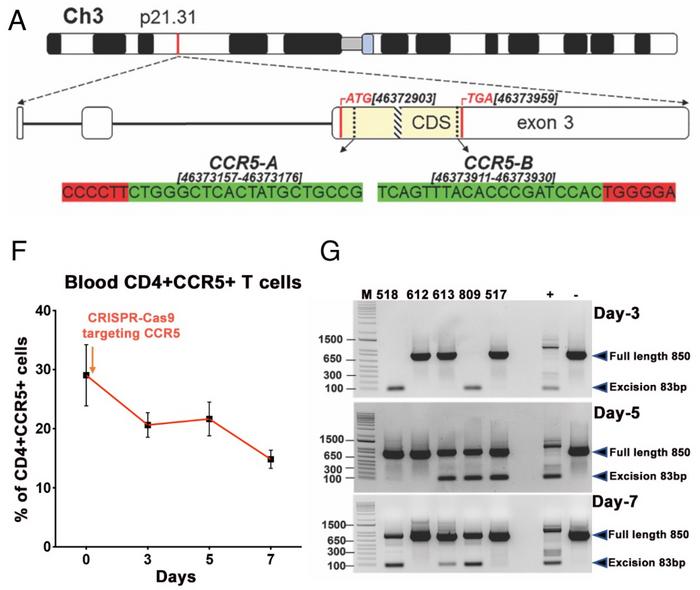
In their new paper published in PNAS in May 2023, the team further targeted the host CCR5 receptor with CRISPR. CCR5 is located on the surface of host immune cells and provides a method of entry for the HIV-1 virus to infect the cell. CCR5 is essential for HIV infection, as demonstrated in the clinic in 2007 when the HIV-infected "Berlin patient" received a bone marrow transplant from a healthy donor with a Δ32 deletion in CCR5. The patient stopped taking ART after the transplant, but even so, levels of HIV plummeted to undetectable levels.
Kamel Khalili and Howard Gendelman deployed the dual CRISPR strategy by first infecting humanised mice with HIV and then treating them with LASER ART for four weeks (see Figure 1). One week later, mice were injected intravenously in the tail vein with CRISPR-Cas9 targeting CCR5 delivered by adeno-associated virus serotype 6 (AAV6).
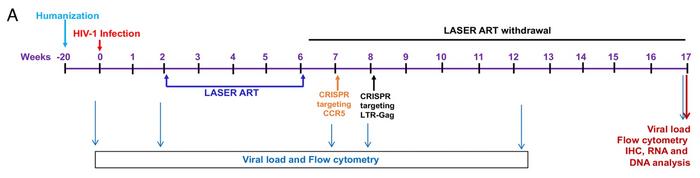
Two gRNAs targeting each side of the Δ32 deletion region were included, and initial experiments had shown that gene editing led to the expected truncation of CCR5 and reduction of CCR5+ T cells (see Figure 2). No off-target cleavage at the top 10 in silico-nominated off-target sites in the human genome could be detected. These data indicated that CRISPR targeting of CCR5 could interfere with HIV spread and potentially reduce the number of integrated viral genomes. This is important for the second round of CRISPR that was initiated one week later, which was aimed at excision of the proviral DNA from the host genome.
Here, CRISPR-Cas9 and two gRNAs targeting excision of the HIV-1 LTR-Gag region were delivered by AAV9 (another AAV serotype was used to avoid immunological reactions). The procedure was the same as reported in the 2019 paper, and again, no cleavage of predicted off-target sites was detected.
Timing must be surgically manipulated
The two CRISPR treatments were performed one week apart, and that was important to do so, Howard Gendelman explains:
» It's important because even if we excise CCR5, new cells will come from the bone marrow and replenish CCR5, allowing new target cells to be infected by HIV. So, we have a narrow window of less than two weeks to eliminate CCR5 before excising HIV from already-infected cells. So, the timing of CCR5 elimination and HIV excision had to be almost surgically manipulated.«
The results showed that in the absence of previous ART treatment, the dual CRISPR strategy was ineffective and led to a continuous decline in CD4+ cells (see Figure 3). With ART, however, the dual CRISPR strategy kept CD4+ cell levels steady, as did the ART plus CRISPR-LTR-Gag treatment. ART plus CRISPR-CCR5 resulted in a modest decline in CD4+ cells from Week 12 to 17.
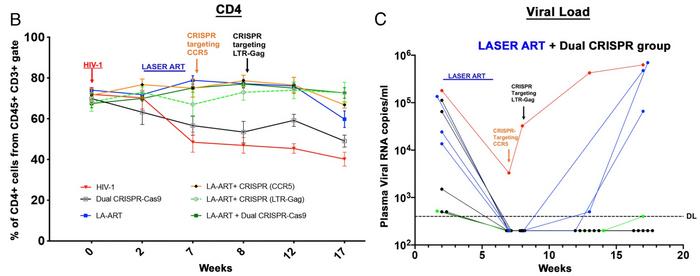
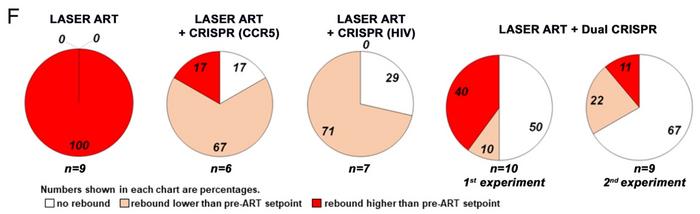
Viral load analyses showed that 50% of animals treated with ART and dual CRISPR had no detectable virus throughout Week 17. At the same time, HIV could barely be detected in one more animal in this treatment group. No detectable virus at Week 17 was also observed for 29% of animals receiving ART plus CRISPR-LTR-Gag and 17% receiving ART plus CRISPR-CCR5. Statistical analysis showed a significant effect of the dual CRISPR treatments (p = 0.00006).
The experiment was repeated for nine more animals in the ART plus dual CRISPR group to validate the results. In this case, no virus could be detected in 67% of the animals. Pooled data for the two experiments showed that HIV could not be detected in 58% of animals in the ART plus dual CRISPR group (see Figure 4).
To affirm these data, viral DNA and RNA levels in various tissues of infected mice from the first experiment were analysed. Both tests showed no detectable viral nucleic acids in 60% of animals in the dual CRISPR group. The same was true in 29% and 16% of animals in the single CRISPR groups (HIV-1 and CCR5, respectively).
The ultimate goal is a one-shot cure
»HIV can hide in many cell types, so we analysed several tissues - spleen, gut, bone marrow, lung, liver, kidney, and brain - with highly sensitive digital droplet PCR, or functionally by adoptive transfer to see if any viruses came up. So, we're confident that the dual CRISPR therapy targeting both the viral genome and the CCR5 receptor eliminates HIV to the point that even without ART, the virus will not rebound,« says Kamel Khalili.
“I think a combination therapy that includes MOGS, CCR5 and HIV excision can take us where we want to be. Our ultimate goal is a one-shot cure for HIV in humans”Kamel Khalili
He cautions, however, that these experiments are performed in humanised mice and need to be replicated in larger animals, like a simian immunodeficiency virus (SIV) model for HIV in macaques.
While the pair continue to investigate the potential of dual CRISPR therapy for HIV-1, the first patient has already been dosed in a clinical trial based on the CRISPR strategy described in several cell and small and large animal studies performed during the last nine years since the creation of CRISPR for elimination of HIV-1. That candidate therapy, EBT-101, which is being developed by U.S.-based Excision Bio Therapeutics (co-founded by Kamel Khalili), removes HIV proviral DNA from infected cells using CRISPR-Cas9 and two or more gRNAs. And there is more to come.
In May 2023, Kamel Khalili published a paper in Molecular Therapy Nucleic Acids describing yet another strategy for HIV elimination called CRISPR-MOGS. Here, CRISPR is used to inactivate the host mannosyl oligosaccharide glucosidase (MOGS), which is critically important for the morphogenesis of virions and viral entry. The paper demonstrates that the inactivation of MOGS in infected cells results in the production of non-infectious virus particles and leads to the diminution of HIV-1 infection in CD4+ T cells.
»I think a combination therapy that includes MOGS, CCR5 and HIV excision can take us where we want to be. Our ultimate goal is a one-shot cure for HIV in humans,« concludes Kamel Khalili.
Link to the original article in PNAS:
CRISPR editing of CCR5 and HIV-1 facilitates viral elimination in antiretroviral drug-suppressed virus-infected humanized mice (2023) Proc. Natl. Acad. Sci. USA 120: e2217887120
For more information, see:
Long-acting slow effective release antiretroviral therapy (2017) Expert Opin. Drug Deliv. 14: 1281
Sequential LASER ART and CRISPR Treatments Eliminate HIV-1 in a Subset of Infected Humanized Mice (2019) Nat. Commun. 10: 2753
Strategic self-limiting production of infectious HIV particles by CRISPR in permissive cells (2023) Mol. Ther. Nucleic Acids 32: P1010
To get more of the CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.

The First CRISPR Medicine Conference, Copenhagen April 2024 - More details soon...
Tags
ArticleInterviewNewsAdeno-associated virus (AAV)Human Immunodeficiency Virus Infection, HIVCas9Excision BioTherapeuticsClinical
CLINICAL TRIALS
Sponsors:
Suzhou Maximum Bio-tech Co., Ltd.
Sponsors:
Zhejiang University