Antibody-Targeted Lipid Nanoparticles Reach High Cancer Therapy Efficacy With CRISPR-Cas9
CMN Webinar: CRISPR Delivery Systems
"Targeted CRISPR/Cas9 Nanomedicine for Therapeutic Genome Editing in Cancer"
On-demand available here.

For years, Dan Peer from Tel-Aviv University in Israel has been working with RNA precision medicine, and he has been a pioneer in RNA delivery strategies. His group recently published a paper in Science Advances, describing a very efficient system for CRISPR-Cas9 cancer therapy using a novel lipid nanoparticle (LNP) delivery system. The system was tested in mice that had been inoculated with two of the deadliest cancers, glioblastoma and metastatic ovarian cancer, which are associated with five-year survival rates of 7% and 47%, respectively. And the therapy achieved ground-breaking results.
»One of the most fascinating aspects of this study was that we were able to increase the survival after glioblastoma by 30% after a single injection of the treatment. Moreover, the survival after metastatic ovarian cancer increased by 80% with double injections. This has a great impact on minimising the treatment regimen and improving patients’ quality of life,« says co-first author Anna Gutkin, who is a PhD student in the Peer Lab.
“One of the most fascinating aspects of this study was that we were able to increase the survival of glioblastoma by 30% after a single injection of the treatment. Moreover, the survival of metastatic ovarian cancer increased by 80% with double injections”Anna Gutkin
CRISPR RNAs are Delivered by Lipid Nanoparticles
CRISPR-Cas9 is a promising technique to improve cancer treatment by enhancing T cell reactivity or disrupting cancer survival genes. The technology has great potential for in vivo applications, but the choice of a suitable delivery vehicle is a critical factor that must address the large size of the CRISPR reagents and the risk of allergic or other adverse reactions. As an alternative to the widely used viral vectors, lipid nanoparticles (LNPs) have attracted great interest as they are clinically approved for RNA delivery and can be extensively modified to improve desired characteristics.
With Peer’s expertise in LNPs, they were chosen as the preferred delivery vehicle, but before assembling them, the team also had to decide in what form Cas9 should be delivered. Daniel Rosenblum, who is a postdoc in Peer’s Lab and joint first author on the recent paper explains:
» There are multiple advantages of using Cas9 in an mRNA format as opposed to plasmid DNA. Many studies have demonstrated that the shorter the exposure time to the Cas9 nuclease, the lower the off-target rate. By using Cas9 mRNA, the exposure time is limited, and moreover, there is no risk for genomic integration, which improves the safety profile.«
New Lipids had to be Developed
The researchers selected four ionisable amino lipids from a library screen and evaluated their performance as LNPs with Cas9 mRNA and sgRNA. To stabilise the RNAs and minimise their immunogenicity, they were chemically modified with 5-methoxyuridine and proprietary methods before being combined with the lipids in a microfluidic mixer.
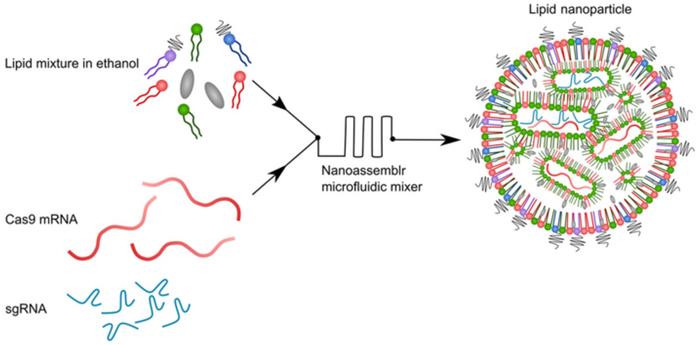
Encapsulation efficiency and biophysical properties of the LNPs containing CRISPR reagents (from here on named cLNPs) were established before evaluating their gene disruption potential. This was done by encapsulating GFP sgRNA and using the resulting cLNPs in GFP-expressing human embryonic kidney (HEK293) cells. In this assay, non-GFP fluorescing cells were counted by flow cytometry and used as a measure of gene disruption efficiency. Based on all these tests, one lipid - Lipid 8 - was chosen for further study and it was then mixed with three structural lipids to form the final cLNPs. Among other characteristics, cLNPs based on Lipid 8 achieved 98% on-target modification and <0.1% modification of a non-target control gene.
»The biggest challenge of this study was to develop an efficient and specific delivery method for both Cas9 mRNA and sgRNA in a single vehicle. The relatively large size of Cas9 poses a great challenge for delivery in both viral and non-viral delivery systems. We had to develop new lipids to enable this efficient delivery,« Rosenblum says.
The choice of lipids is critical for the function of cLNPs. Since the nanoparticles are supposed to travel through the cell membrane, the charge of the lipids has to be carefully controlled, and they also have to be biodegradable. Yizhou Dong, who did not participate in the study, leads a research group at Ohio State University and is an expert in drug delivery. He recently developed a series of biodegradable lipid-like nanoparticles suitable for mRNA-delivery, and he is impressed by the functionality of the new cLNPs from the Peer Lab.
»It’s very exciting that the lipid nanoparticles reported by Peer and colleagues can effectively deliver gene editing components into mouse glioblastoma cells after intracerebral injection. Moreover, editing of the PLK1 gene led to significant inhibition of tumour growth in the animal models,« says Dong.
Disruption of PLK1 Specifically Kills Cancer Cells
The GFP disruption assay turned out to be really helpful during the development of the delivery platform, so the team decided to also use it in the subsequent experiments. Accordingly, GFP-expressing cancer cell lines were established – namely murine glioblastoma (GBM) and human ovarian adenocarcinomas (OV8). The GBM cell line was chosen due to its high proliferation rate, high invasiveness, and infiltrative nature that resemble human tumours. Similarly, the OV8 cell line behaves like advanced stage human tumours by metastasising throughout the peritoneal cavity and by forming malignant ascites.
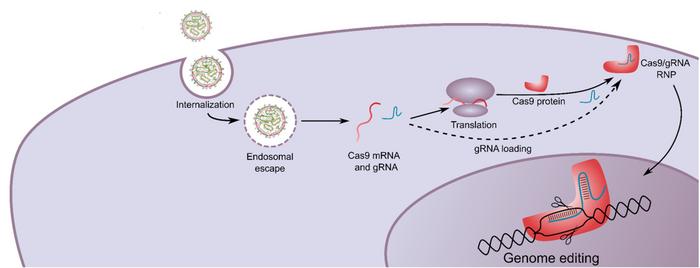
For these experiments, the researchers used cLNPs packed with PLK1 sgRNA rather than GFP sgRNA. PLK1 is a kinase required for mitosis, and its disruption leads to cell cycle arrest and cell death in dividing cells, while non-dividing cells are spared. Gutkin explains:
»PLK1 expression is very low in normal cells. Therefore, we do not expect high toxicity to normal cells by targeting PLK1 for disruption. However, we used PLK1 as a proof of concept of our delivery platform, and other tumour-specific genes can also be used to improve specificity.«
sgPLK1-cLNPs Potently Increase Brain Cancer Survival
To explore their potential for cancer therapy, sgPLK1-cLNPs were first incubated with GBM and OV8 cancer cells in vitro. This disrupted PLK1 in 84% and 91% of the cells, respectively, and reduced cell viability after four days 5- and 10-fold, respectively. Next, potential toxicity and immunogenicity were evaluated by injecting wild-type mice with sgGFP-cLNPs. These efforts revealed no significant differences of liver enzyme levels, blood counts, or cytokine levels.
In the paper from Peer’s lab, the authors state that this was only an initial safety evaluation and that more extensive evaluation of potential toxicity is needed for preclinical development. Yizhou Dong agrees and says:
»It would be helpful to establish a safety profile for these nanomaterials for future clinical translation for cancer therapy or other therapeutic indications.«
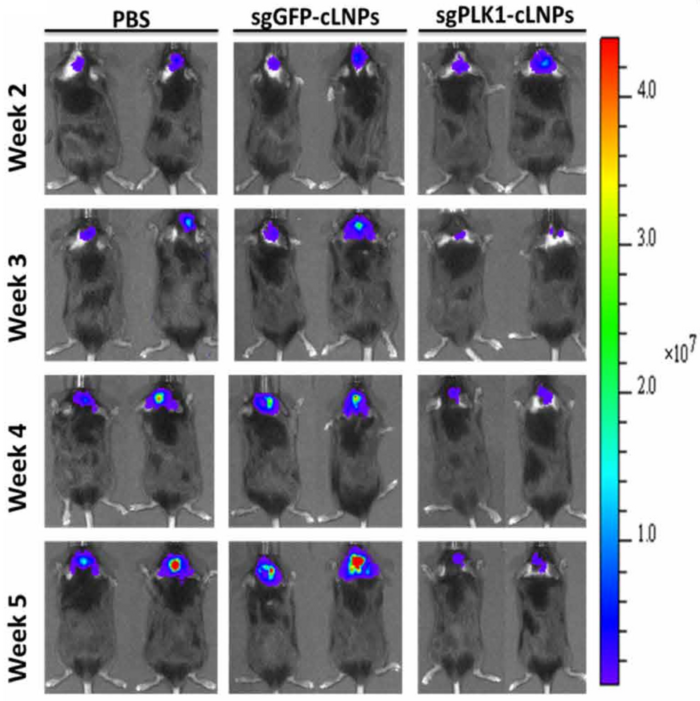
Though a more robust safety evaluation is eventually needed, the team moved on to explore the efficacy of the system in mice that had been injected with GBM brain cancer cells into the hippocampus. After 10 days, bioluminescence imaging confirmed that tumours were established, and therapeutic sgPLK1-cLNPs were injected into the tumour bed. Two days later, mice were euthanised, and next-generation sequencing revealed that 68% of cancer cells had been edited at the PLK1 locus. After three days, staining for caspase-3 - a marker of apoptosis - indicated that cell death was occurring only in mice treated with sgGFP-cLNPs.
“As far as we are aware, these findings represent the highest survival improvement in this aggressive tumour after a single treatment”Dan Peer
More importantly, the treatment also translated into reduced brain tumour growth and increased survival. Bioluminescence imaging revealed significantly slower tumour growth in sgPLK1-cLNP-treated animals compared to controls, and tumours even started to shrink after six weeks. Moreover, treated animals significantly outlived controls. When the experiment was terminated after 60 days, 30% of sgPLK1-cLNP-treated animals were still alive, while all of the control mice had died by day 40. These results indicate an increase in median survival by around 50% and an improvement of overall survival by 30%.
»As far as we are aware, these findings represent the highest survival improvement in this aggressive tumour after a single treatment,« Dan Peer and the other authors write in the paper.
Antibody Targeting is Essential for Metastatic Tumours
The researchers then tried out the cancer therapy in mice that had been inoculated with OV8 ovarian cancer cells. In this case, there was no or minimal effect of injecting sgPLK1-cLNPs 10 and 17 days after tumour inoculation. This didn’t come as a surprise, however, as Rosenblum explains:
» In the GBM mouse model, cLNPs were injected directly into the tumour bed. Therefore, non-targeted cLNPs enter the tumour cells surrounding the injection site very efficiently. In the OV8 model, however, cLNPs were injected systemically. As the metastatic tumours are spread throughout the peritoneal cavity, untargeted cLNPs hardly reach these tumours.«
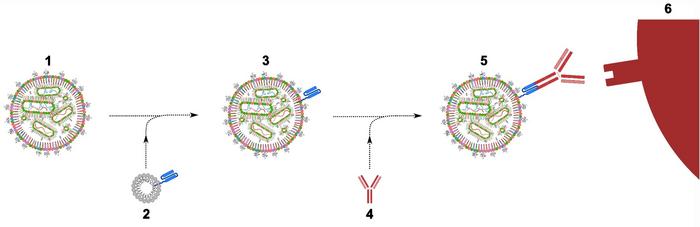
To solve this problem, the team turned to a flexible method for antibody-targeted cell-specific delivery of RNAs that the Peer Lab developed in 2018. The method comprises a lipid-anchored single-chain antibody linker that enables easy switching of the targeting antibody and thereby provide high target cell versatility. Using this system, sgPLK1-cLNPs were decorated with antibodies targeting the epidermal growth factor receptor (EGFR) that is abundantly expressed on OV8 cancer cells.
With this modification, the cancer therapy had a significant effect as revealed by fluorescence imaging. Cancer cells hardly grew during the first five weeks after treatment in contrast to controls where tumours increased by an order of magnitude in the same period. Survival rates also increased dramatically with two injections of EGFR-targeted sgPLK1-cLNPs. When the experiment was terminated after 60 days, all control animals as well as 90% of those receiving untargeted treatment had died. However, only 10% of animals receiving treatment targeted to EGFR-expressing cancer cells had died at this timepoint. These results translate into an increase of overall survival of around 80% with EGFR-targeted sgPLK1-cLNPs.
The New CRISPR-LNPs platform is Heading for the Clinic
The antibody-targeting system adds to the safety of the CRISPR-based cancer therapy developed by the Peer Lab since it improves cancer cell specificity and reduces unintended editing in normal cells. And the team has great expectations for the future.
» We are currently evaluating additional cancer-killing target genes. Also, we are looking at ways to expand our system to other malignancies as well as to rare genetic diseases such as Duchenne muscular dystrophy,« says Gutkin, and Rosenblum adds:
»We really hope to take our CRISPR-LNPs platform to the clinic in the next few years.«
Link to the original article in Science Advances:
https://advances.sciencemag.org/content/6/47/eabc9450
Tags
ArticleNewsDeliveryDelivery - BrainIn vivoLipid-based nanoparticleCancerSolid TumoursGene therapyCas9
CLINICAL TRIALS
Sponsors:
Suzhou Maximum Bio-tech Co., Ltd.
Sponsors:
Zhejiang University