Cellectis Bets on Non-Viral Editing for Therapy Gains
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
Gene therapy's viral vector problem – effective delivery but manufacturing complexity and safety concerns – has sent companies searching for alternatives. Cellectis reports that circular single-stranded DNA (cssDNA) combined with TALEN achieves over 40% gene insertion in haematopoietic stem cells whilst delivering five-fold better engraftment of corrected cells compared to adeno-associated virus approaches in murine models.
“In a field that has been dominated by CRISPR for the ease of access to the technology, marked by rapid expansion among private and academic stakeholders and a corresponding increase in patent litigation driven by financial interests, Cellectis occupies a differentiative position”Julien Valton, Cellectis
In an interview following the publication of a Nature Communications study in November last year, Julien Valton, Vice President Gene Therapy at Cellectis, outlines how the non-viral approach addresses the manufacturing scalability and therapeutic durability challenges facing the field.
Cellectis' commitment to TALEN technology represents a contrarian bet in a CRISPR-dominated landscape. Valton frames the strategic rationale by quoting Adam Bogdanove, who helped decipher TAL effector DNA recognition: »TAL effectors revolutionized the field of DNA targeting, CRISPR democratized it.«
Strategic positioning in a CRISPR world
»Cellectis has dedicated the past 26 years to designing, developing, and optimizing a range of gene editing technologies, including notably Meganuclease, CRISPR-Cas9, and TALEN,« Valton explains. »Through this extensive experience and know-how, we have generated a wide variety of synthetic DNA-binding modules powered by algorithms. We have determined that, when carefully designed, optimized, and selected, a TALEN beats other gene editing technology and more particularly CRISPR-based approaches for therapeutic applications.«
The claimed superiority rests on precision and specificity. »TALEN constructs can be designed for every seven base pairs across the entire genome, with knockout efficiencies ≥90% in various cell types and loci, with minimal to no off-sites detected,« he notes. »These features have enabled us to generate universal CAR T-cells, successfully treating the first pediatric patients more than ten years ago. To that note, these patients treated in 2015 have been the first patients ever treated by gene-edited cells.«
Valton positions the accumulated intellectual property as a competitive moat: »In a field that has been dominated by CRISPR for the ease of access to the technology, marked by rapid expansion among private and academic stakeholders and a corresponding increase in patent litigation driven by financial interests, Cellectis occupies a differentiative position. Fifteen years of improvement to harness TALEN technology, developing extensive assets and know-how, generating and maintaining a robust and comprehensive intellectual property portfolio, Cellectis is exceptionally well positioned to leverage its platform – together with the non-viral cssDNA vectorization strategy – to advance the development of next-generation ex vivo and in vivo cell and gene therapy products.«
Solving the stem cell retention problem
The latest study addresses a fundamental bottleneck, namely therapeutic gene insertion into quiescent long-term haematopoietic stem cells (LT-HSCs), the population that determines durable treatment outcomes. Traditional homology-directed repair works efficiently in dividing cells but falters in these primitive, non-dividing cells.
»Retention of therapeutic knock-in events in transplanted edited HSPCs is of utmost importance to ensure their long-term therapeutic benefit in patients. However, achieving this goal has long been daunting for scientists using engineered nucleases to insert a therapeutic DNA donor template at a precise endogenous locus,« Valton explains. »Our results suggest we can bypass this hurdle by demonstrating efficient gene insertion in quiescent long-term HSCs. This feature represents a clear advantage to improve the therapeutic potential of edited HSPCs in the field of gene therapy.«
The research employed a dual electroporation protocol with TALEN mRNA followed by cssDNA templates (see Figure 1). This achieved up to 49% gene insertion with 2.2 kilobase templates across multiple genomic loci. Single-cell transcriptomic analysis revealed cssDNA editing generates ten-fold higher levels of gene-corrected LT-HSCs compared to AAV-mediated editing. In sixteen-week murine engraftment studies, cssDNA-edited cells showed five-fold higher engraftment of gene-corrected cells despite similar initial editing levels.
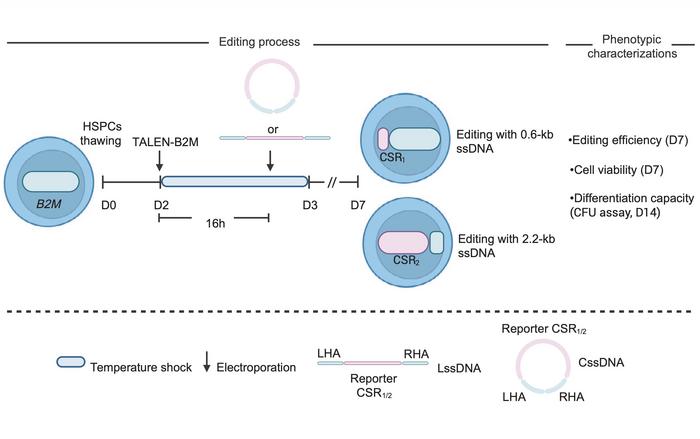
The edited cells retained a quiescent metabolic state and expressed elevated bone marrow adhesion markers, including CXCR4, CD44, and F11R.
»The quiescent status of edited HSPCs implied to us that the non-viral gene insertion process maintained the pool of long-term HSCs and delayed or mitigated their differentiation into progenies,« Valton notes. »The presence of high levels of common bone marrow adhesion markers at their surface confirms our hypothesis and indicates edited HSPCs are equipped to efficiently engraft.«
Manufacturing scalability as a differentiator
The DNA donor template is a critical manufacturing parameter, particularly for autologous therapies that require patient-specific production. Valton emphasises this strategic consideration: »When it comes to ex vivo cell therapy development, considering every single step of manufacturing is of utmost importance. One of them is the sourcing of the DNA donor template. Its origin, GMP compliance, and manufacturing scalability represent three parameters that led us to select Moligo's technology to produce the single-stranded DNA used in our study.«
“TALEN constructs can be designed for every seven base pairs across the entire genome, with knockout efficiencies ≥90% in various cell types and loci, with minimal to no off-sites detected”Julien Valton, Cellectis
The cssDNA production employs enzymatic synthesis via rolling circle amplification, an entirely in vitro process. »Moligo has developed an enzymatic DNA synthesis technology based on rolling circle amplification that is entirely performed in vitro, enabling the synthesis of circular single-stranded DNA longer than 10 kb and with any level of sequence complexity. Their technology, Enzymatic Injection Molding Technology, can achieve yields up to three orders of magnitude higher than fermentation-based methods,« Valton explains.
»It does not require the use of helper phages or helper plasmids and does not leave any scar sequences, unlike plasmid-based production. It does not include any PCR steps, relying only on isothermal in vitro enzymatic reactions, which makes the process highly scalable,« he continues. »Moreover, the absence of bacterial in vivo amplification, similarly to in vitro mRNA vaccine production, avoids endotoxin contamination, which can affect fermentation-based approaches used by other single-stranded DNA manufacturers.«
The compact, well-controlled process enables deployment near cell therapy manufacturing facilities whilst simplifying GMP compliance – practical advantages for commercial-scale production.
Expanding therapeutic applications
The ability to insert multi-kilobase templates determines which therapeutic strategies become feasible. Valton outlines the practical scope: »We demonstrate in this work that the length of most therapeutic DNA templates used for gene therapy – whether employing ex vivo viral-mediated editing tools such as lentiviral vectors or adeno-associated virus – is comparable to that of the cssDNA template (2–3 kb) utilized in our study. Therefore, we believe our non-viral editing approach could be applied to address most of the indications currently pursued by the gene therapy community, including X-linked severe combined immunodeficiency, mucopolysaccharidosis type I, Wiskott–Aldrich syndrome, chronic granulomatous disease, or severe pyruvate kinase deficiency, as a series of examples of a non-exhaustive list.«
The research also demonstrated approximately 40% gene insertion in primary T cells using the same platform, positioning the technology for CAR T-cell applications.
Valton concludes with an ambitious outlook: »We talk about a true revolution to come in molecular medicine in the next decade and a game changer for the way patients are going to be treated, addressing not only the symptoms but also the roots of diseases.«
Whether this translates to clinical success depends on upcoming development milestones, but the combination of TALEN precision, cssDNA manufacturing scalability, and preserved stem cell function provides a technical foundation for therapeutic programmes whilst potentially avoiding some safety and manufacturing challenges that have complicated viral vector approaches.
Link to the original study in Nature Communications on 19 November 2025:
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







