Clinical Trial Update: Encouraging Safety and Efficacy Data From Phase 1 TALEN Trial in Multiple Myeloma
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
UNIVERSAL, sponsored by Allogene Therapeutics, is a dose escalation trial of ALLO-715 in patients with heavily pretreated multiple myeloma (MM). All patients enroled in the trial have undergone at least three prior lines of MM therapy, and all were refractory to their last line of treatment.
Interim results from the ongoing Phase 1 UNIVERSAL study published in Nature Medicine last week includes data from the first 48 patients enrolled in the trial, with a data cutoff of October 2021. The results suggest that ALLO-715 provides clinical benefit while reducing the lengthy time-to-treatment associated with conventional autologous CAR-T therapy.
ALLO-715 safety profile consistent with CAR-T cell therapy and lymphodepletion
Safety and tolerability were assessed in 43 patients who received lymphodepletion with ALLO-647 (an anti-CD52 antibody), fludarabine and cyclophosphamide (FCA), followed by 1 of 4 escalating doses of ALLO-715, a gene-edited anti–B cell maturation antigen (BCMA) AlloCAR T™ cell therapy.
Cytokine release syndrome, a common side effect of CAR-T cell therapy that includes fever and low blood pressure, was observed in 24 patients (55.8%), with 1 grade ≥3 event (2.3%). Infections occurred in 23 patients (53.5%), with 10 (23.3%) of grade ≥3. Six patients (14%) experienced neurotoxicity, with no grade ≥3 events. In total, 38 patients (88.0%) reported grade ≥3 AEs.
At the optimal dosing regimen (FCA lymphodepletion followed by 320 × 106 CAR+ T cells), 17 of 24 patients (70.8%) had a clinical response, including 11 (45.8%) with very good partial response or better and 6 (25%) with a complete response/stringent complete response. The median duration of response was 8.3 months.
Previously, data from the UNIVERSAL study were presented at Allogene Therapeutics R&D Showcase in November 2022, and the American Society of Hematology Annual Meeting in December 2022.
An allogeneic CAR-T cell candidate that targets BCMA
B cell maturation antigen (BCMA) is a member of the tumor necrosis factor receptor family, and is one of the most specific and highly expressed antigen on myeloma cells.
BCMA-targeted treatments have an established role in MM, including two FDA- and EMA-approved autologous anti-BCMA chimeric antigen receptor (CAR)-T cell-mediated treatments: idecabtagene vicleucel (Abecma, Bristol Myers Squibb) and ciltacabtagene autoleucel (CARVYKTI, Janssen Biotech, Inc.).
Unfortunately, creating patient-specific autologous CAR-T cells is a costly and time-consuming process involving cell harvesting, gene modification and cell expansion, which can delay treatment by several weeks.
Allogeneic CAR-T cells can be synthesised in large batches and stored for later use, thereby reducing cost and treatment delays. However, immunocompetent donor cells recognise patient cells as “foreign” and mount an immune response against healthy patient cells leading to a serious condition known as graft vs host disease (GvHD). Likewise, the patient’s immune system mounts an attack against donor cells, including CAR-T cells, leading to their rejection.
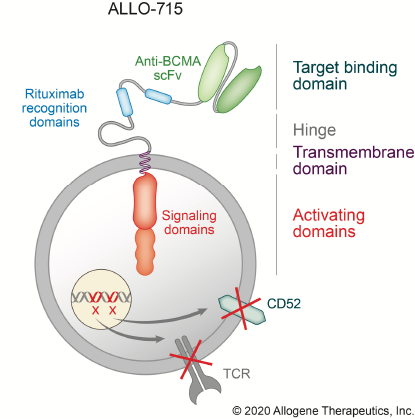
Overcoming allotype immune response between donor and patient cells
Allogene Therapeutics’ approach is to circumvent CAR-T cell rejection and GVHD using TALEN gene editing to knock out 2 critical genes, TCRα subunit constant gene (TRAC) and CD52.
GvHD is driven by donor recognition of host cells through the αβ T cell receptor complex (αβTCR); knocking out TRAC eliminates αβTCR-mediated GvHD.
The glycoprotein CD52 is expressed on immune cells and promotes T cell activation and proliferation; blocking CD52 enhances lymphodepletion and reduces rejection of CAR-T cells but also reduces CAR-T cell proliferation and viability. When used with CD52-edited CAR-T cells, ALLO-647 (a humanised anti-CD52 mAb) selectively depletes host T cells while protecting donor cells.
The UNIVERSAL trial was initiated in 2019 and data collection was scheduled to complete in December 2022. Additional results from the estimated enrolment of 132 patients, along with reporting of patients treated with ALLO-715 in combination with the selective gamma protease inhibitor Nirogacestat are highly anticipated. We will continue to share updates on the UNIVERSAL trial as they emerge.
For a complete overview of current gene editing clinical trials, check out CRISPR Medicine News' Clinical Trials Database.
To get more of the CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Karl Torbey MD writes about science and medicine and is based in the United States.
Tags
ArticleNewsClinical News UpdatesCancerMultiple Myeloma, MMCancerCAR-TTALENsAllogene Therapeutics, Inc.
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







