Clinical Update: CRISPR-Edited Checkpoint Cell Therapy for Gastrointestinal Cancer
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
New York-based Intima Bioscience is sponsoring a first-of-a kind trial that explores gene-edited immune checkpoint therapy for Stage IV metastatic gastrointestinal cancers. The principle investigator is gastrointestinal oncologist Emil Lou at the Masonic Cancer Center at the University of Minnesota (UMN).
The trial, which is still recruiting, is open to adults with Stage IV metastatic gastrointestinal epithelial cancer who have had at least one first line standard therapy and who are in reasonably good health. So far, two patients have been treated without evidence of acute toxicity related to the CRISPR-edited TILS (see Abstract 208), and a total of 20 adult participants are expected to be enrolled and treated by October 2022.
CISH inactivation boosts the activity of tumour-fighting immune cells
The trial was initiated following groundbreaking research by scientists at National Cancer Institute (NCI) and UMN last year, who found that CRISPR-Cas9-mediated inactivation of the cytokine-inducible SH2 (CISH) gene in tumour infiltrating lymphocytes (TILs), increased their capability to fight solid tumours. We previously described these findings in detail in an interview with Douglas Palmer (NCI), Beau Webber and Branden Moriarity (both UMN).
TILs are a naturally occurring subset of immune cells that migrate from blood into tumours. The idea behind the new therapy, which combines CRISPR and the principle of CAR T-cell therapy, is that CISH inactivation in TILs and other T cells will augment their natural ability to target and destroy solid tumours, opening up a new treatment avenue for solid tumours, which often respond poorly to CAR-T cell therapies.
Cytokine-inducible SH2 (CISH)
CISH is a novel cancer immune checkpoint that is believed to be a suppressor of tumour immunity via negative regulation of T cell receptor (TCR) signalling. Unlike the widely discussed checkpoint protein PD-1 that resides on the cell surface, CISH is expressed intracellularly and biophysically proximate to the T cell receptor (TCR) signalling complex. The location of CISH makes it a poor target for cell surface-targeting antibodies, but CRISPR provides a solution to this challenge.
Clinical-scale GMP manufacturing of CISH-disrupted primary human TILs
The new therapy is developed from autologous (patients’ own) cells, which are isolated from patient tumour tissue. From this, TILS are isolated and then edited with CRISPR to remove the CISH gene. The edited cells are expanded in the lab with the help of the interleukin 2 (IL-2) cytokine, before being infused back into the patient following lymphodepletion, as depicted in the schematic below. Lymphodepletion is a treatment that reduces the patient’s own T cell numbers, so they will not antagonise the proliferation of the infused engineered T cells.
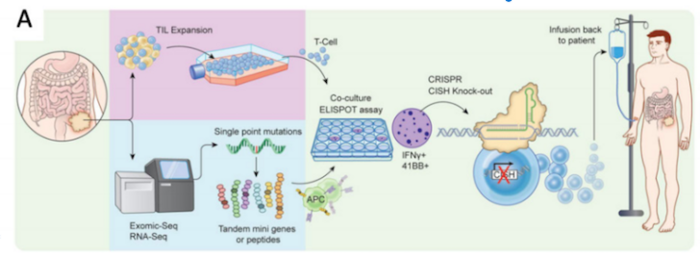
TILs are considered challenging to isolate from tumours and are usually isolated in very small numbers. This requires successful in vitro expansion to achieve the cell titers necessary for treatment. Despite a tricky manufacturing process, scientists behind the new therapy reported success in clinical-scale GMP compliant manufacturing at the annual American Society of Gene & Cell Therapy (ASGCT) meeting, which was held last month (see Abstract 208).
They report > 90% CISH gene disruption and >95 % CISH protein reduction without detectable off-target editing as measured using amplicon sequencing of predicted off-target loci and unbiased GUIDE-Seq in CISH-edited cells. The viability and expansion capacity of edited cells was described as high and CISH-knockout cells exhibited increased cytokine-dependent proliferation, T cell receptor (avidity) and neo-antigen recognition, as compared to non-edited cells.
We will continue to bring updates as they emerge. In the meantime, you can read more about the thoughts and planning behind this trial in our interview with Emil Lou just after the trial opened last year.
For a complete overview of current gene editing clinical therapeutic trials as well as diagnostic trials, check out CRISPR Medicine News' Clinical Trials Database.
Tags
ArticleNewsin vivoEsophageal CancerGastro-Intestinal Cancer, GISolid Tumor AdultSolid TumoursCancerCas9Intima Bioscience, Inc.TrialsClinical
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







