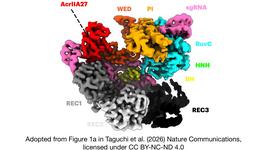
CMN Weekly (10 November 2023) - Your Weekly CRISPR Medicine News
By: Gorm Palmgren - Nov. 10, 2023
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
Top picks
- The Innovative Genomics Institute's Affordability Task Force, established in 2022, is tackling the high costs of CRISPR genomic therapies to improve access and healthcare stability. Their recent publication in Nature's Gene Therapy journal outlines a roadmap for affordable treatments, addressing challenges like manufacturing costs and business models.
- Researchers in Columbia have provided in vivo evidence of the potential of a CRISPR-nCas9-based gene therapy strategy for treating mucopolysaccharidosis IVA (MPS IVA) using iron oxide nanoparticles (IONPs) as non-viral vectors. The authors inserted the human GALNS cDNA into the ROSA26 locus in an MPS IVA mouse model. They showed increased GALNS activity, mono-KS reduction, partial recovery of the bone pathology, and non-IONPs-related toxicity or antibodies-mediated immune response activation.
Research
- American scientists have successfully used prime editing to correct the c.1222C>T mutation in the PAH gene, a common cause of phenylketonuria (PKU), in vitro and in a mouse model. This breakthrough, achieving 52% liver-wide correction, resulted in the complete normalization of phenylalanine levels, indicating its potential as a treatment for PKU patients.
- Base-editing is another approach for therapeutic correction of the same mutation as above. American researchers found that upon delivery of a selected adenine base editor mRNA/guide RNA combination into a mouse model via lipid nanoparticles (LNPs), there was sufficient PAH editing in the liver to normalize blood Phe levels within 48 h fully.
- American researchers have used systematic epigenetic CRISPR screening to identify BATF3 as a critical regulator of the transcriptional and epigenetic state of human CD8+ T cells. Overexpression of BATF3 thus enhances the therapeutic potential of these cells in adoptive T-cell therapies, particularly for cancer treatment.
- Swiss researchers have used CRISPR screening to identify mechanisms of resistance to KRASG12C and SHP2 inhibitor combinations in non-small cell lung cancer. They found that KRASG12C amplification and MAPK/PI3K pathway alterations were predominant resistance mechanisms to combined KRASG12C/SHP2 inhibitors in preclinical settings.
Industry
- Vor Bio has presented updated clinical data from patients treated in VBP101, its Phase 1/2a multicenter, open-label, first-in-human study of trem-cel (VOR33) in patients with acute myeloid leukaemia (AML). Primary neutrophil engraftment occurred in all seven patients treated with trem-cel.
- Synthego has announced the launch of IND-enabling (INDe) gRNAs, a transformative product that revolutionizes the CRISPR-based cell and gene therapy preclinical pipeline. This innovative offering empowers researchers with high-quality gRNAs designed specifically for GLP-regulated preclinical and IND-enabling studies. It incorporates comprehensive IND-compliant materials and documentation in their design and production, facilitating a seamless transition from preclinical to clinical applications.
Third quarter 2023 financial results and business updates
- Verve Therapeutics ended the third quarter of 2023 with $485.2 million in cash and net losses of $45.8 million.
- Caribou Biosciences ended the third quarter of 2023 with $396.7 million in cash and net losses of $10.0 million.
- Intellia Therapeutics ended the third quarter of 2023 with $992.5 million in cash and net losses of $122.2 million.
- Vor Bio ended the third quarter of 2023 with $160.1 million in cash and net losses of $33.2 million.
- Iovance Biotherapeutics ended the third quarter of 2023 with $427.8 million in cash and net losses of $113.8 million.
- Cellectis ended the third quarter of 2023 with $72 million in cash and no mention of net losses.
- Prime Medicine ended the third quarter of 2023 with $178.8 million in cash and net losses of $50.7 million.
- Fate Therapeutics ended the third quarter of 2023 with $349.7 million in cash and net losses of $45.2 million.
- Precision BioSciences ended the third quarter of 2023 with $122.2 million in cash and net losses of $8.1 million.
- Poseida Therapeutics ended the third quarter of 2023 with $238.8 million in cash and net losses of $31.8 million.
- Vertex Pharmaceuticals ended the third quarter of 2023 with $13,600 million in cash, while net gains are not shared. However, the overall financial performance indicates positive growth, with increased revenues and net income despite increased expenses and investments.
- CRISPR Therapeutics ended the third quarter of 2023 with $1,739.8 million in cash and net losses of $112.2 million.
- Editas Medicine ended the third quarter of 2023 with $446.4 million in cash and net losses of $45.0 million.
- Beam Therapeutics ended the third quarter of 2023 with $1.000 million in cash and net losses of $96.1 million.
Detection
- Chinese researchers have developed a rapid and sensitive method for detecting Pseudomonas aeruginosa by isothermal amplification combined with Cas12a. The method can detect as low as 50 CFU/mL, and the whole detection process can be finished within one hour.
- Researchers in China present a rapid, sensitive detection method for SARS-CoV-2 and influenza virus based on recombinase polymerase amplification combined with CRISPR-Cas12a. The detection system is highly specific, and there is no cross-reactivity with other common respiratory tract pathogens.
Reviews
- Mechanistic toxicology in light of genetic compensation. This review discusses mechanisms by which short insertion and deletion mutations in one gene may be rescued by compensatory activity. The authors argue that a premature termination codon inserted by gene editing will not cause a loss of functional protein, and they provide guidelines for using CRISPR to generate mutations that avoid compensation.
- Loop-mediated isothermal amplification-integrated CRISPR methods for infectious disease diagnosis at point of care. This review discusses LAMP-integrated CRISPR-based nucleic acid detection methods in point-of-care (PoC) pathogen detection platforms and identifies current limitations and future directions.
- CRISPR/Cas12-based electrochemical biosensors for clinical diagnostic and food monitoring. This review discusses the application of CRISPR-Cas12-based electrochemical biosensors, as well as various electrode modifications that have been successfully used to improve the performance of these biosensors in the clinical and food monitoring fields.
Perspectives
- A perspective in Biopharma Dive discusses why the gene editing therapy developed by Vertex Pharmaceuticals and CRISPR Therapeutics may be more complex than its dramatic benefit suggests. One major problem is the necessary preparatory chemotherapy regimen before exa-cel is infused, which is so arduous that older people and those with organs damaged by sickle cell may not be healthy enough to receive it.
- An article in The Conversation examines the prospects of using gene editing to cure HIV. The discussion is seen in the light of recent positive clinical results of EBT-101 - a CRISPR-based infusion developed by Excision BioTherapeutics.
- A piece in BioPharm International urges contract development and manufacturing organizations (CDMOs) to embrace the potential of CRISPR. It argues that CRISPR is essential for the future of cell line development and for meeting the ever-increasing demands of the fast-growing global biotherapeutics industry.
Webinars
- On Wednesday, November 22, CRISPR Medicine News is holding a free webinar entitled "Physiological roles of aberrant DNA methylation in vitro and in vivo" by Gabriella Ficz at the Barts Cancer Institute, London. She has used CRISPR technology to investigate the interplay between DNA methylation and cancer. Her latest findings reveal the heritability of artificially induced disease-specific methylation patterns in primary cells. These mechanisms might contribute to cancer initiation and hint at future therapeutic and preventative avenues.
- Our latest webinar - A Dual CRISPR Strategy Eliminates HIV in Humanised Mice - is now available on demand. It features Professors Kamel Khalili and Howard Gendelman, who are determined to develop a one-shot cure for HIV-1 and use CRISPR to attack the virus on multiple fronts.
News from CRISPR Medicine News
- This Monday, we brought a Startup Spotlight focusing on Ariya Bio. In the ETH Innovation & Entrepreneurship Lab in Zürich (Switzerland), Ariya Bio aims to develop safe and effective gene-editing therapies for genetic diseases. We spoke with two co-founders, Mandy Boontanrart and Jan Nelis, to hear more about Ariya Bio and its unique approach to treating sickle cell disease.
To get more of the CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
CLINICAL TRIALS
IND Enabling
Phase I
Phase II
Phase III
Gastric Cancer and Colorectal Cancer, CRC, (NCT07166263)
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
IND Enabling
Phase I
Phase II
Phase III
Relapsed or Refractory Acute Myeloid Leukemia, AML, (NCT06541444)
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
IND Enabling
Phase I
Phase II
Phase III