CMN Weekly (23 September 2022) - Your Weekly CRISPR Medicine News
By: Karen O'Hanlon Cohrt - Sep. 23, 2022
Top picks
- Excision BioTherapeutics announced in a recent press release that it has dosed the first participant in a Phase 1/2 trial evaluating EBT-101 as a potential cure for HIV. EBT-101 is designed to excise HIV proviral DNA using CRISPR-Cas9 and two guide RNAs that target three sites within the HIV genome, thereby cutting out large portions of the HIV genome and minimising potential viral escape. Read more about EBT-101 in a previous CMN clinical trial update.
- Don't forget to sign up for the next CMN webinar. Our invited speaker, Subhash C. Pandey, PhD, Joseph A. Flaherty, MD Endowed Professor of Psychiatry and Director, Alcohol Research Center, University of Illinois at Chicago, USA will present his work on the topic of 'Epigenomic Editing Ameliorates Detrimental Effects of Adolescent Alcohol Exposure'. Sign up to attend the online webinar here.
Research
- In an article published yesterday in Nature Chemical Biology, researchers at various institutions in the U.S. report using a novel strategy to engineer the Leptotrichia wadei (Lwa)Cas13a by inserting different RNA-binding domains into a unique active-site-proximal loop within its nucleotide-binding domain. Two of the resulting LwaCas13a variants exhibited enhanced collateral activity and improved sensitivity compared to wild-type LwaCas13a in various buffer conditions. The team also demonstrated that the new variants could detect the SARS-CoV-2 genome at attomolar concentrations from inactive viral- as well as unextracted clinical samples, without target preamplification, when used in combination with an electrochemical method.
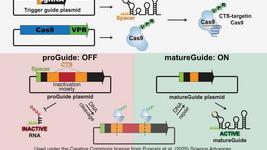
- In an article published in Biochemistry earlier this week, scientists in Denmark comment on two new transposon-associated RNA-guided mechanisms, considering their potential as new gene-editing solutions. The authors firstly focus on a group of small RNA-guided endonucleases of the IS200/IS605 family of transposons, which likely evolved into class 2 CRISPR effector nucleases (Cas9s and Cas12s), and then address the co-option of the RNA-guided activity of different CRISPR effector nucleases by a specialised group of Tn7-like transposons to target transposon integration. Read the full details in the article here.
Clinical
- Intellia Therapeutics recently announced positive interim clinical data for its second in vivo investigational CRISPR candidate, NTLA-2002, for the treatment of hereditary angioedema. NTLA-2002 is designed to knock out the kallikrein B1-encoding gene in hepatocytes to permanently reduce plasma kallikrein activity and hence halt the production of the inflammatory mediator bradykinin, which is dependent upon plasma kallikrein, and which is overproduced in HAE. The NTLA-2002 clinical trial data revealed thata single dose of NTLA-2002 led to a 65% and 92% mean plasma kallikrein reduction at doses of 25 mg and 75 mg, respectively, at week eight following treatment. Read more about the mechanism of NTLA-2002 here.
- Intellia Therapeutics and Regeneron have announced initial data from the cardiomyopathy arm of the ongoing Phase 1 trial of NTLA-2001, an investigational single-dose in vivo CRISPR-Cas9 therapy for the treatment of transthyretin (ATTR) amyloidosis. Read more about the available clinical data for NTLA-2001 in a previous CMN clinical trial update here.
Industry
- Verve Therapeutics has announced clearance of its application for clinical trial authorisation to the UK Medicines & Healthcare Products Regulatory Agency for VERVE-101 in patients with heterozygous familial hypercholesterolemia. VERVE-101 is a novel, investigational base-editing medicine designed to be a single-course treatment that permanently turns off the PCSK9 gene in the liver to reduce disease-driving low-density lipoprotein cholesterol (LDL-C). Read more about VERVE-101 in our recent interview with Verve CEO Sekar Kathiresan here.
- Avectas, an Irish cell-engineering technology business and GenScript, a life-sciences research tools and services provider, announced earlier this week that they are partnering to develop an improved non-viral cell therapy manufacturing process. According to a press release, the goal of the partnership, which will combine Avectas' cell-engineering technology and expertise with GenScript's experience in synthetic long oligo production, is to improve the editing efficiency and cell viability of non-viral based cell therapies.
- Earlier this week, Beam Therapeutics presented the first pre-clinical in vivo data demonstrating the potential of a multiplex base-editing approach to reduce viral markers, including hepatitis B surface antigen expression, and prevent viral rebound of hepatitis B virus in animal models of disease. The data were presented at the INSERM Cancer Research Center of Lyon in France.
- Ring Therapeutics, a U.S.-based life sciences company aiming to revolutionise gene therapy with its commensal virome platform, announced earlier this week the issuance of a U.S. patent for its Anellovector™ compositions. The patent covers anellovirus vectors, which can be used to deliver a diversity of therapeutic modalities, including therapeutic polypeptides or nucleic acids.
Reviews
- Immune Responses to Gene Editing by Viral and Non-Viral Delivery Vectors Used in Retinal Gene Therapy. In this review, researchers in France evaluate studies that have reported on pre-existing immunity to ocular gene therapies, and discuss both innate and adaptive immune responses with a specific focus on immune responses to gene editing, both with non-viral and viral delivery in the ocular space. The authors finish with a discussion of the approaches available to prevent and manage immune responses to ensure safe and efficient gene editing in the retina.
- Therapeutic Applications of the CRISPR-Cas System. The authors of this review focused on medical applications of CRISPR-Cas and discuss the trends and limitations, with an emphasis on CRISPR-based therapeutics for infectious disease, oncology, and genetic disease, as well as CRISPR-based diagnostics, screening, immunotherapy, and cell therapy.
- A comprehensive overview of CRISPR/Cas 9 technology and application thereof in drug discovery. This review presents the current status of CRISPR-Cas technology in a tailor-made format from its discovery to several advancements for drug discovery, alongwith future trends associated with possibilities and hurdles including ethical concerns.
Opinion and comments
- After early wins, CRISPR gene editing is about to get a lot harder. This piece in STAT looks at what the future might have in store for the application of CRISPR gene editing in humans.
Awards
- The Feinberg School of Medicine and Simpson Querrey Institute for Epigenetics has awarded its inaugural Kimberly Prize in Biochemistry and Molecular Genetics to biochemist and Nobel laureate Jennifer Doudna. The Kimberly Prize is the largest biochemistry award in the U.S., worth $250,000, and the award to Doudna was announced by Northwestern on September14th.
News from CRISPR Medicine News
- For this week's feature article, we spoke with medical doctor Silja Hansen, who is currently a PhD student at Aarhus University in Denmark. She discusses her current research project and the promise, challenges, and future of prime editing to treat inherited retinal diseases. Read the interview here.
Huh, Heh, Wow
- An international research team from KeyGene and Wageningen University & Research, both in the Netherlands, and Leibniz Institute of Plant Biochemistry in Germany, have developed chicory plants that accumulate costunolide, a plant metabolite known for its anti-cancer activity. The team used CRISPR-Cas9 to block further processing of costunolide into other plant metabolites, resulting in the accumulation of the medical compound. The findings of the work were published recently in Frontiers in Plant Science.
To get more of the CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
CLINICAL TRIALS
IND Enabling
Phase I
Phase II
Phase III
Gastric Cancer and Colorectal Cancer, CRC, (NCT07166263)
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
IND Enabling
Phase I
Phase II
Phase III
Relapsed or Refractory Acute Myeloid Leukemia, AML, (NCT06541444)
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
IND Enabling
Phase I
Phase II
Phase III