Creating new AAV vectors for gene therapy

Adeno-associated virus (AAV) vectors are the vector of choice for in vivo delivery of genetic material into cells, including gene editing tools such as CRISPR-Cas9. These vectors are efficient, are considered safe, and several gene therapies based on AAV delivery have already been approved - Glybera (AAV1), Luxturna (AAV2) and Zolgensma (AAV9).
Despite all of their assets, wild-type AAVs require improvement with respect to their ability to effectively and/or selectively target tissues and cells. This is because the specificity of naturally-occurring AAVs is often too broad, enabling them to infect several different cell types in parallel. As well as this, some cell types are refractory to known AAVs.
Now, German researchers have published a system for spitting out a variety of new and improved AAVs to target many cell types effectively.
»In most instances, we found an AAV suitable for targeting the cell type our collaborators or we were interested in,« says first author Kathleen Börner from the Medical Faculty, Department of Infectious Diseases/Virology and BioQuant Center, Heidelberg University in Germany.
Her primary focus is HIV. Thus, she initially set out with Hans-Georg Kräusslich’s and Dirk Grimm’s groups at the university to find an AAV that could deliver CRISPR reagents into blood cells.
»When I started the project, the aim was to evolve or identify AAVs to transduce T-cells and macrophages, which are poorly transduced by wild-type AAVs,« says Börner.
Peptide display
To find new AAV delivery vectors, she used a system based on the principle of viral peptide display that can be harnessed by any biology lab. Originally, members of the Grimm lab introduced small peptides into a loop on the surface of the viral capsid. Each peptide alters the natural interactions between the virus and the cell, and, ideally, simultaneously repurposes and retargets the AAV to new tissues or cell types. The read-out is a functional transduction assay using a YFP reporter gene (Yellow Fluorescent Protein) that is encoded in the AAVs genome. A fluorescence reader measures the amount of fluorescence emitted by the cells, and from this, it is possible to calculate the number of transduced cells.
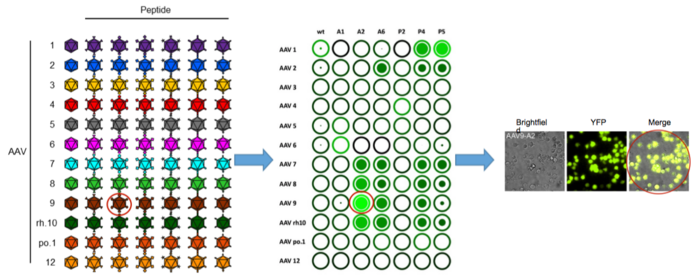
Exactly how peptide insertion affects the viral life cycle is difficult to predict. Besides the initial binding to the cell, the peptides can also influence subsequent steps such as intracellular transport and traficking to the nucleus.
»We only modify the virus slightly, and it's not informed, so we don't know what the outcome will be. However, we can rapidly find out by screening all our variants in a given cell type in high throughput,« says Börner.
Searchable database
Surprisingly, peptide display technology has been around for about 15 years, but until now it has been predominantly used in combination with the AAV2 serotype.
Therefore, in the new study, Kathleen Börner and colleagues systematically took 13 different AAVs and inserted 27 peptides in their capsids.
It turns out that the combination of different AAV serotypes and peptides is an excellent approach, and the fluorescent reporter screens described above reveal that it is possible to find a vector for over 90 cell types of interest.
Notably, all of the results from this work have been made available in a searchable online database (see box).
Database SPIRIT
The SPIRIT (Superior Peptide InseRtions for Improved Targeting) database is a collection of data that Börner and colleagues obtained from screening the AAVs on different cell types, and this is freely accessible online.
Users can search for cell types that may be transduced by an AAV, and they can also exclude those which the AAV should not transduce to identify specific variants.
Since AAV is very stable and can be stored, plates with pre-aliquoted capsid variants can be shipped to researchers whose favourite cell type may be missing in the database and who want to test the AAVs in their own lab.
Once in a lifetime
So the system can produce AAV delivery tools for a large variety of cell types, but how specific is the delivery?
This is impossible to predict but it can fortuntaely be tested by screening multiple cell types in parallel. Most of the AAVs tested to date have not exhibited cell-type specificity as the researchers found them to be active in different cell types. However, during another study carried out in the Grimm lab, Jonas Weinmann and colleagues found an AAV peptide variant that specifically transduces muscle tissues in mice, including the diaphragm and heart.
»We were very lucky and surprised because the discovery of such a unique capsid may only happen once in a lifetime,« says Dirk Grimm.
Targeting muscle cells
In line with her original research, Börner now hopes to use several of the identified AAVs against the HIV.
One specific idea is to harness the 'muscle-targeting' AAV for expression of a neutralising antibody against HIV and to inject it systemically. The virus would then find its way to the muscle tissue and deliver its cargo to the cells.
»The myocytes [muscle cells, -ed.] would express and secrete the antibody, and you would have an immune prophylaxis against HIV,« says Kathleen Börner.
»The basic concept has been published, but the combination of this particular AAV with the neutralising antibodies will be new.«
This is only one of many possible avenues. For example, another exciting option would be a CRISPR-Cas9 gene editing therapy to treat muscular dystrophy. To do this, one would inject the AAV encoding the Cas and the guide RNA, akin to a recently published study in a Duchenne muscular dystrophy murine model.
Do your RNAi or your CRISPR
Börner sees the new AAV collection as a basis for many future projects.
»We were positively surprised by how well the different AAVs work, especially considering the small production scale.« says Kathleen Börner.
She also points out the ease with which the system can be used in any lab equipped for cell culture under the lowest biosafety level 1.
»It has already kicked off many projects, which required an efficient gene delivery tool and began with this kind of screen. Once you have identified a lead candidate that is suitable for the on-target cell, you can immediately use it to express RNAi, CRISPR or any other transgene you want as long as it fits into the AAV.« says Börner.
Tags
CLINICAL TRIALS
Sponsors:
Suzhou Maximum Bio-tech Co., Ltd.
Sponsors:
Zhejiang University