CRISPR Epigenetic Editing Delivers Durable Multiplexed Gene Silencing in T Cells
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
Current CAR-T cell therapies have transformed treatment for certain blood cancers, but substantial advances are needed to tackle solid tumours and develop off-the-shelf allogeneic products. While CRISPR-Cas9 genome editing has become a predominant tool for engineering therapeutic T cells, it relies on introducing DNA double-strand breaks that can result in permanent genomic changes and potentially harmful chromosomal abnormalities. Alternative strategies using transcriptional editors like CRISPRi and CRISPRa can perturb gene expression without DNA damage. Still, these require sustained expression of bacterial Cas proteins, which trigger immune recognition and rejection of transplanted cells.
“CRISPRoff gene silencing is maintained through numerous cell divisions, T cell stimulations and in vivo adoptive transfer, avoiding cytotoxicity or chromosomal abnormalities inherent to multiplexed Cas9-mediated genome editing”Goudy et al.
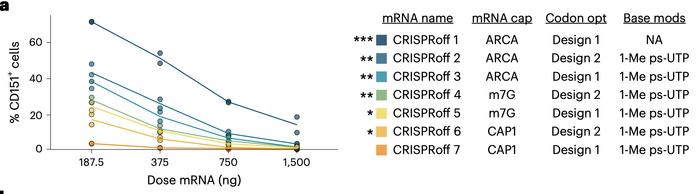
The research team optimised an all-RNA delivery system for CRISPRoff, an epigenetic editor composed of catalytically inactive Cas9 fused to DNA methyltransferase domains DNMT3A, DNMT3L and the KRAB domain from ZNF10. They systematically compared seven mRNA variants differing in cap structure, base modifications and codon optimisation. The most potent design, designated CRISPRoff 7, incorporated Cap1 structure, 1-methylpseudouridine substitution and a specific codon optimisation algorithm, achieving complete silencing in 85 to 99% of primary human T cells at high mRNA doses with no observed toxicity (see Figure 1).
To establish the durability of epigenetic silencing, the researchers compared CRISPRoff with CRISPRi and Cas9 nuclease across multiple endogenous gene targets over 28 days. While CRISPRi-mediated silencing was progressively lost over time – particularly upon T cell restimulation – CRISPRoff programmed durable gene silencing comparable to Cas9 knockout. The silencing persisted through three rounds of anti-CD2/CD3/CD28 antibody restimulation, demonstrating stable propagation across approximately 30 to 80 cell divisions in vitro. RNA sequencing confirmed highly specific targeting, with robust repression of intended genes and no off-target differentially expressed genes at 28 days after electroporation. Whole-genome bisulfite sequencing revealed that the highest differentially methylated region between targeting and control samples occurred precisely at the targeted locus.
The platform proved effective across diverse genomic contexts. For genes with CpG islands, including therapeutically relevant targets such as FAS, PTPN2, RC3H1, SUV39H1, MED12 and RASA2, at least one guide RNA achieved potent and durable silencing. The team also successfully targeted genes lacking CpG island annotations, achieving stable silencing of CD5 and LAG3 that remained 99.5% and 99.1% silenced, respectively, at 30 days, whereas PDCD1 showed 78% silencing in bulk populations.
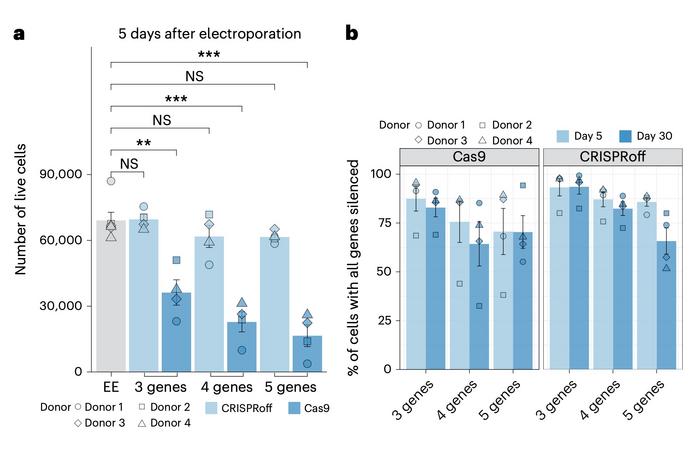
A critical advantage emerged when targeting multiple genes simultaneously. Targeting three, four or five genes with nuclease-active Cas9 resulted in substantial cellular toxicity, likely due to multiple DNA breaks. In contrast, CRISPRoff multiplexed targeting produced minimal toxicity while achieving combined silencing of three, four, and five target genes at 93.5%, 82.4%, and 65.8% efficiency, respectively, across four donors at 30 days.
»CRISPRoff gene silencing is maintained through numerous cell divisions, T cell stimulations and in vivo adoptive transfer, avoiding cytotoxicity or chromosomal abnormalities inherent to multiplexed Cas9-mediated genome editing,« the researchers note in the manuscript.
“Epi-edited CAR-T cells can maintain stable target gene silencing even through multiple rounds of successful antigen-positive cancer cell killing, enabling functional enhancement of CAR-T cells through silencing of 'checkpoint' genes without the need for multiplexed gene cleavage”Goudy et al.
Beyond gene silencing, the team developed CRISPRon, a complementary tool comprising catalytically inactive Cas9 fused to the TET1 DNA demethylase domain. They demonstrated that targeting CRISPRon to the Treg-specific demethylated region (TSDR), an intronic enhancer at the FOXP3 locus, could remove endogenous repressive DNA methylation in conventional CD4-positive T cells. This resulted in constitutive FOXP3 expression that remained stably upregulated over 28 days in culture, contrasting with previous attempts that showed rapid remethylation. Targeted bisulfite sequencing confirmed reduced methylation specifically at the TSDR in targeted cells.
The clinical potential of this technology was validated through integration with CAR-T cell manufacturing. The researchers developed orthogonal CRISPR systems using Acidaminococcus species Cas12a ribonucleoproteins for targeted CAR knock-in at the TRAC locus, combined with CRISPRoff-mediated silencing of RASA2, a gene whose ablation promotes T cell function across immunosuppressive conditions. This combined approach maintained efficient CAR integration whilst achieving robust gene silencing. In repetitive stimulation assays, RASA2-silenced CAR-T cells continued to kill target cells efficiently after five rounds of stimulation. In contrast, control-edited cells progressively declined in their ability to control cancer cells.
The enhanced functionality translated to improved outcomes in preclinical models. In NSG mice bearing Nalm6 leukaemia, animals treated with RASA2-silenced CAR-T cells showed significantly better tumour control and extended survival compared to control CAR-T cells across multiple independent experiments and four donors.
»Epi-edited CAR-T cells can maintain stable target gene silencing even through multiple rounds of successful antigen-positive cancer cell killing, enabling functional enhancement of CAR-T cells through silencing of 'checkpoint' genes without the need for multiplexed gene cleavage,« the authors emphasise.
Importantly, CRISPRoff-induced silencing remained stable following in vivo adoptive transfer. When CD151-silenced CAR-T cells were transferred into mice bearing subcutaneous A375 melanoma tumours, highly efficient knockdown was maintained in CAR-T cells isolated from tumours and spleens 14 days after transfer, confirming that epigenetic silencing persists through tumour antigen recognition and the in vivo environment.
Laine Goudy and Luke Gilbert led the study from the University of California, San Francisco and Arc Institute. It was published in Nature Biotechnology on 21 October 2025.
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleCMN HighlightsNewsStickyEpigenome editing (e-GE)dCas9
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







