CRISPR Makes Resistant Lung Cancer Cells Vulnerable to Chemotherapy
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...

Many types of cancer can be treated with relative ease, but this is not the case for late-stage non-small cell lung cancer (NSCLC). There is a pervasive sense of hopelessness among these patients owing to the current lack of treatment options, therapeutic failure due to drug resistance, and low survival rates. It was this sense of despair that drove researchers from ChristianaCare’s Gene Editing Institute to develop an innovative CRISPR therapy that could make chemotherapy-resistant NSCLC cells vulnerable to standard chemotherapy once again.
In an article published last month in Gene Therapy, the team describe a new anti-cancer strategy that uses CRISPR-Cas9 to knock out Nuclear Factor Erythroid 2-Related Factor 2 (NRF2), a protein that is overexpressed in NSCLC cells and leads to chemoresistance. With a strong focus on safety and the patient experience, principal investigator Kelly Banas Ph.D. left no stone unturned - this study clearly demonstrates that there are knockouts, and then there are knockouts.
The paper is unique because of its deep dive into the spectrum of outcomes that result from CRISPR knockouts of NRF2. With this approach, the team were able to develop a robust strategy that is likely to considerably improve patient outcomes; with careful design, CRISPR-based disruption of NRF2 can result in exon skipping, which in some cases may increase chemosensitivity in cancer cells. As senior author Eric Kmiec Ph.D. sees it, this strategy will not only be applicable to other types of cancer, but also to the broader CRISPR field:
»This is a platform-based approach to all kinds of solid tumours. We’re committed to understanding the mechanism and regulation of CRISPR at all levels through foundational, truthful science. That’s how the field will gain credibility.«
Know thy enemy: NRF2
Kelly Banas knows NRF2 like the back of her hand, and for good reason. In addition to the present study, it was the topic of her doctoral dissertation. It was this in-depth knowledge of NRF2 that allowed the team to design an effective CRISPR knockout strategy with a level of precision not normally seen in knockout studies.
NRF2 is a master regulator of hundreds of other genes that are involved in many cytoprotective and metabolic pathways. Under normal physiological conditions, NRF2 protects cells from oxidative stress, toxic insult, and chemotherapy, keeping the cells in homeostasis.
»Cancer cells hijack the NRF2 pathway and dysregulate that function. When NRF2 becomes overly expressed and accumulates within the cell, the cell is able to fight off these toxic insults. That’s really where the cancer becomes chemoresistant. Later-stage patients have much higher levels of NRF2,« Banas explains.
NRF2’s key role in the progression of cancer made it an obvious target for CRISPR-based disruption. The team’s goal was to provide better outcomes for the late-stage NSCLC patients for whom treatment options are limited, by knocking out NRF2 to make the cancer cells vulnerable to chemotherapy. To test their strategy, they used two relevant cell lines: human lung adenocarcinoma A549 cells and human lung squamous cell carcinoma NCI-H1703 cells, delivering CRISPR reagents as a ribonucleoprotein (RNP) complex.
»Chemotherapy is traditionally used for stage 3 NSCLC and people become resistant to it early on. You want to knock NRF2 out [in cancer cells] because it’s a stress-response protein. The cell goes into survival mode and shuts itself off. If you destroy NRF2, it can’t avoid the toxic stress of chemo. Chemotherapy is traditionally used for stage 3 NSCLC and people get resistant to it early on - it’s really about the cell’s ability to withstand stress,« Kmiec comments.
An interesting aspect of the team’s research is that in a couple of the tumour types they investigated, NRF2 possesses a unique PAM site that does not exist in the genomes of healthy cells. Using this to her advantage, Kelly Banas was able to demonstrate 99% cleavage activity in the tumour genome and 0% in the normal genome, i.e., of healthy control cells. This unique PAM exists in a small but significant percentage of tumours, including squamous cell carcinoma, providing an exciting opportunity to use the tumour’s DNA against itself while eliminating possible off-target editing.
Not all knockouts are created equal
As Banas and her co-authors describe in their article, gene knockouts are anything but simple. In fact, even with purposeful design, gene knockout can result in a myriad of outcomes, each of which can have a significant impact on the response to treatment.
»A transcription factor like NRF2 needs to enter the nucleus. We could [delete] a binding domain, but the protein could still get into the nucleus and wreak havoc. That’s why we went after the nuclear entry signal. I think that’s something that can be missing in the field that could be causing inconsistent results. People think if there is a PAM site somewhere, that’s where they should knock the gene out, but they need to think about functional domains, « Kmiec says.
A key discovery of the study is that CRISPR-based disruption of NRF2 can result in exon skipping. A dual sgRNA approach to target exon 4 – which codes for the nuclear entry signal – resulted in deletion of a 103-base pair fragment, including exonic splicing enhancer sequences.
»We started out targeting NRF2 to knock it out at the genetic level. What we saw was frameshifting indels in the genome, but this wasn’t enough to completely knock it out. At that point, we looked at the mRNA and protein levels to get a better understanding of what was going on. Then we saw these exon skipping events happening,« Banas describes.
Although the mechanism behind CRISPR-induced exon skipping has not been fully elucidated, previous papers have described similar exon skipping events as a result of CRISPR edits.
»Depending on where you’re targeting, you can lose essential splicing sites that are necessary to transcribe, regulate and modify the mRNA downstream,« Banas adds.
Exon skipping became the focus of the study because of its effect on chemosensitivity of the cells. Banas discovered that depending on which exons are skipped and which functional domains of NRF2 remain, the response to chemotherapy differs. Even a single nucleotide difference in the genome of NRF2-knockout cells can mean the difference between exon skipping occurring or not, based on the impact on exonic splicing enhancer sequences. Of course, characterising the huge range of outcomes of CRISPR edits was a major challenge, as Kmiec explains:
»This was an inordinate amount of work. We were looking at over 400 clones that Dr. Banas had to isolate individually, on 96 well plates, and then grow them all. But she told us in a few lab meetings that something weird was happening and the protein was being truncated, and because we’re committed to the truth, we said, ‘Go find it’. Dr. Banas had the courage of her convictions and her training to pursue this and figure it out,« Kmiec says.
Exon skipping may regulate NSCLC response to chemotherapy
After performing the CRISPR edits and examining the range of outcomes at the genomic, transcriptomic, and protein levels, Banas and the team used a cell viability and proliferation assay to assess the response of various clonal populations to treatment with the drug cisplatin. Cisplatin is a common chemotherapy agent and is part of the standard regimen for NSCLC.
As they expected, the responses of the different clonal populations of NRF2-disrupted cells varied considerably, but exon skipping was not a major factor in the outcome that would impact clinical application. In particular, cells in which exon 4 of NRF2 was skipped displayed heightened sensitivity to cisplatin, because this exon encodes the nuclear entry signal that allows NRF2 to function.
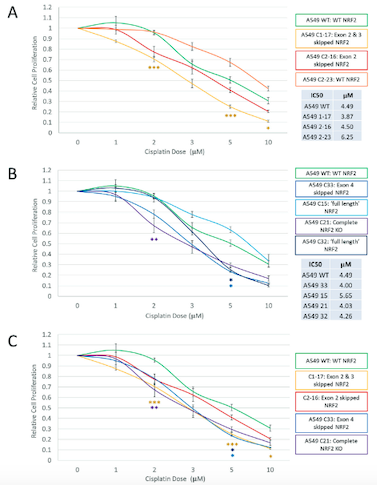
»By targeting those functional domains, we’re eliminating that function of NRF2 and the cell responds to chemo,« Banas elaborates. The increased sensitivity of particular clonal populations was exactly the result they had hoped for when beginning the work. However, exon skipping does not always lead to positive outcomes.
»Any time you see a physical change in a chromosomal pattern, whether its translocation or exon skipping, it should worry you. When you break DNA, all kinds of crazy things can happen when it's repaired,« Kmiec comments.
One possible outcome of creating double-stranded breaks in a gene is that the chromosome attempts to preserve the protein by simply truncating it. This is problematic because the resulting truncated protein could be antigenic and cause an autoimmune response.
»In this study we see heightened sensitivity [due to exon skipping], and it’s accelerated our programme on the clinical side, because we have confidence in it. But this could have gone the other way, where the cells could have gotten more resistant, or the edit could have produced an abnormal protein and caused an autoimmune response - we’re very cautious.« Kmiec adds.
Safety and the patient perspective
The study is, first and foremost, about safety. Kmiec and Banas don’t want to tout CRISPR cures without being able to back up their assertions with positive patient outcomes, particularly for late-stage patients facing poor prognoses.
»For me, being in the field of gene therapy from the beginning, the hype and overpromise have often come because researchers have not thought carefully about their target. CRISPR has been so publicised and popularised, but there is no [FDA-approved] CRISPR-based drug yet. We’re learning as we go,« Kmiec notes.
Around 25% of all lung cancer patients are not treatable with chemotherapy and immunotherapy, and as Kmiec describes it, chemotherapy for these patients has shown little improvement in recent years, with many highly toxic drugs that were developed in the early 1970s still being used today. As such, reducing the amount of chemotherapy for NSCLC patients is a primary goal towards extending survival times.
»Our goal is to provide several months more of life. There’s no reason we should give up on late stage NSCLC patients. We’re realistic about our endpoints, but we want to fight for these people. That’s what ChristianaCare teaches you – to focus on the patient, not just the technology. We’re patient, steady, and methodical,« Kmiec asserts.
Another key focus for the team is providing this type of treatment to the patients who need it most, including those who are mistrusting or skeptical of medical intervention. One of their strategies is to increase the outreach and education activities carried out by the centre.
»In a community cancer centre, we see patients from all different socio-economic levels and cultural backgrounds, which are not always adequately represented in clinical trials. We want to make sure these breakthrough technologies reach minority communities that support what we’re doing – we’re very committed to that. We want to make sure people aren’t left out, and that in the future, people won’t be so afraid of gene editing,« Kmiec says.
“If something’s weird, it’s weird for a reason, and you need to pursue it. We want [CRISPR therapy] to be safe before it’s efficacious. We need to ask why these things are happening and examine the clinical implications. That’s the perfect definition of translational research. If we can relieve people’s suffering at any level, we’ll be successful”Eric Kmiec Ph.D.
Addressing unmet medical needs and moving into the clinic
As the team continues the research in pre-clinical studies, Banas is still following every lead in the search for a safe treatment. This can be challenging given the rapid pace of progress in the CRISPR field.
»The assays change every month. Even when we think we’re using the right techniques to analyse and move this therapy forward, suddenly a new one comes out that’s much better or more robust, so we have to start all over again,« Banas points out.
Kmiec stresses that translational research is much more likely to be successful with the input of surgical residents and patients because they enable researchers to make more informed decisions about the relevance of their strategies.
»We have great insight into the targets and the patient perspective. We’re using the exact same drug and we try to get as close to the relevant dose as possible. It seems like the patient-driven experience is overstated here, but it’s not. If we’re going to have an effect on their clinical outcome, we need to be reproducing these conditions,« Kmiec explains.
In formal meetings with the FDA, Banas and the team have even been asked to expand their range of targets to include deadly oesophageal cancer, based on the promise of their therapeutic technique. The range of outcomes of CRISPR edits demonstrated in this study is a teachable moment for anyone working to develop CRISPR-based therapies.
»If something’s weird, it’s weird for a reason, and you need to pursue it. We want [CRISPR therapy] to be safe before it’s efficacious. We need to ask why these things are happening and examine the clinical implications. That’s the perfect definition of translational research. If we can relieve people’s suffering at any level, we’ll be successful,« Kmiec concludes.
Rebecca Roberts, Ph.D. is a molecular biologist and science writer/communicator based in Queensland, Australia.
Link to the original paper in Gene Therapy:
To get more of the CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







