Droplet cell mechanoporation boosts CRISPR efficiency
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
CRISPR-Cas9 technology has revolutionised genome editing, offering precise, efficient modifications across a wide range of organisms. However, a major hurdle limiting its therapeutic potential lies in the delivery of CRISPR components into target cells.
Existing techniques, such as electroporation and viral vectors, often suffer from issues like low efficiency, toxicity, and undesired immune responses. Electroporation, though widely used, can cause substantial cell death and off-target effects due to high-voltage pulses, while viral vectors pose risks of insertional mutagenesis and immune activation.
Developing safe, non-viral delivery methods that ensure efficient internalisation of CRISPR components into cells remains a significant challenge in advancing genome editing for clinical applications.
The DCP platform employed a microfluidic system that encapsulates cells in droplets, passing them through a microscale constriction to create transient disruptions in the cell membrane. This facilitates the entry of CRISPR-Cas9 ribonucleoprotein (RNP) complexes. The DCP system was tested on several cell types, including the suspension cell line K562 and adherent cells like HEK293T, demonstrating its versatility (see Figure 1).
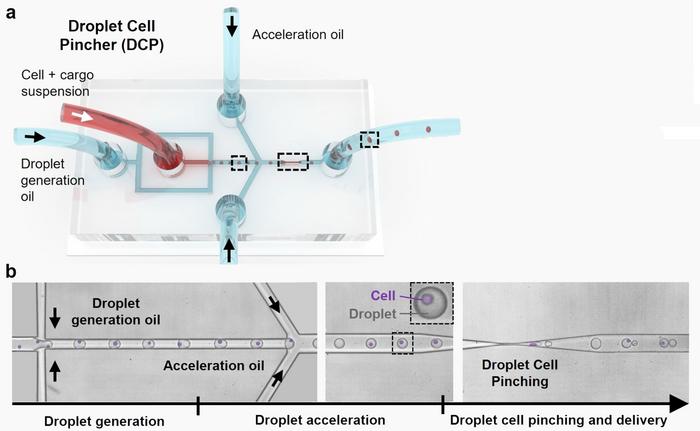
For genome editing, the EMX1 gene was targeted using CRISPR-Cas9 RNP complexes, with guide RNAs designed to direct the Cas9 protein to specific loci. The DCP platform showed remarkable efficiency in delivering macromolecules into the cells, achieving up to ~98% delivery for mRNA and ~91% for plasmid DNA.
The editing performance of the DCP system was compared with electroporation, a common CRISPR delivery method. The DCP platform significantly outperformed electroporation, achieving a 6.5-fold increase in single knockout efficiency and a 3.8-fold improvement in double knockouts (see Figure 2).
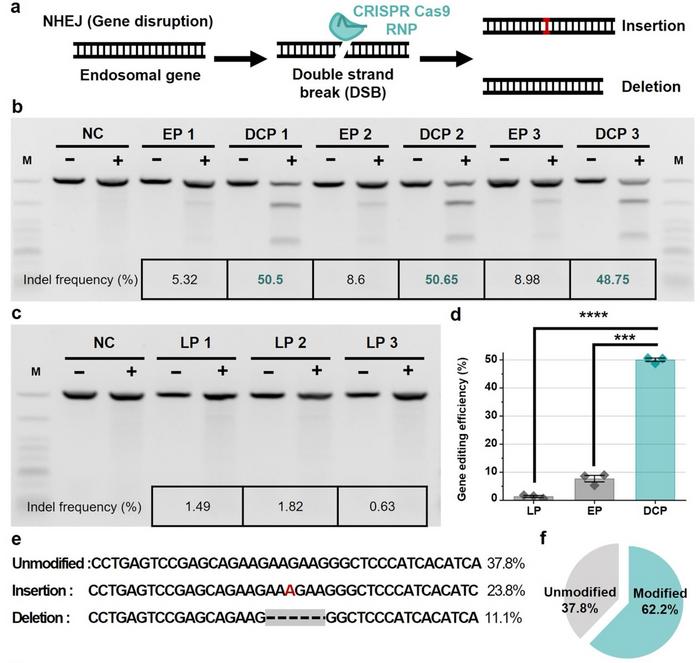
In addition, the system allowed precise knock-ins through homology-directed repair (HDR), with the insertion of donor templates into the EMX1 locus. This was tested by delivering the same RNP targeting EMX1, accompanied by single-stranded DNA (ssDNA) serving as the HDR donor template (112 nt). Results showed that DCP exhibited an insertion efficiency of 14.7%, outperforming electroporation approximately 3.8-fold.
Co-first authors of the study were You-Jeong Kim and Dayoung Yun, while Cheulhee Jung and Aram J. Chung were co-senior authors. The authors are from Korea University and the National University of Singapore. The study was published on 16 September 2024 in Nature Communications.
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleCMN HighlightsNewsDeliveryRibonucleoprotein (RNP)Cas9
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







