Engineered Polymers Show Promise for Systemic mRNA Delivery to Lungs: Potential Implications for Genetic Lung Diseases
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...

Researchers at Johns Hopkins University School of Medicine (Baltimore, MD, USA) developed a novel nanoparticle system that efficiently delivers genetic material to lung cells when administered systemically. This delivery approach is based on engineered biodegradable polymers, which offer several advantages over lipid nanoparticles. Proof-of-concept pre-clinical studies in mice showed high transfection rates in lung cells after intravenous administration, providing a new delivery method for potential gene therapies for genetic lung diseases.
»Although lipid nanoparticles have been used as a delivery method for the treatment of lung diseases, they tend to traffic towards the liver rather than the lungs. This is why we used a different type of polymer, which was found to be a safe and effective method for mRNA delivery,« said Erin W. Kavanagh, the study’s first author. »The nanoparticles also effectively transfected the cell types that are relevant for the treatment of cystic fibrosis and other genetic lung diseases.«
The study was published in Biomaterials.
Unmet need: Efficient delivery of genetic material to lung cells
Genetic lung diseases, particularly cystic fibrosis (CF), are challenging to treat, and often require long-term management. Although gene therapy is potentially curative for CF, delivering therapeutic genetic material to lung cells remains a significant challenge. Most previous gene therapy approaches for CF relied on direct delivery of genetic material to the lungs through inhalation or intratracheal administration, which is impractical and may not reach all affected cells.
Kavanagh explained the motivation behind the study: »The lungs are an important therapeutic target for many diseases. In recent years, we’ve seen an explosion in various nanoparticles. However, many of these nanoparticles have been developed almost exclusively for intratracheal delivery. The delivery challenges are compounded when the mucus in an individual with CF covers the airways, so we wanted to develop a nanoparticle system that could be used for systemic delivery and still achieve high transfection rates in the lungs.«
She added that because CF is ultimately a multi-system disease, it is therapeutically relevant to reach areas outside the lungs, such as the pancreas and intestines.
Approach: Engineering nanoparticles for targeted delivery
Researchers aimed to develop and optimise poly(beta-amino ester)(PBAE) nanoparticles for systemic mRNA delivery. In contrast to lipid nanoparticles, these biodegradable polymers can be easily fine-tuned through chemical modifications to enhance their delivery capabilities.
The team synthesised a library of PBAE polymers with various chemical structures. They used a two-step chemical process to build these polymers, combining different building blocks to create branched structures. These structures included both hydrophilic and hydrophobic parts. To fine-tune the properties of the polymers, the researchers added small molecules to their ends. This process allowed them to create a diverse range of polymers with different characteristics, which they could then test for their ability to deliver genetic material to cells.
Kavanagh highlighted the advantages of PBAEs: »They are able to hold onto nucleic acid so efficiently because they are made up of amine groups that can promote nucleic acid binding and facilitate endosomal escape once they have entered a cell. They also contain hydrolytically-degradable ester groups that allow them to be biodegradable, which reduces the cytotoxicity and aids in RNA or DNA release.«
Researchers screened PBAE polymers for their ability to transfect human bronchial epithelial cells, including cells from patients with CF. This initial screening helped to identify promising candidates for further optimisation, focusing on transfection efficiency, cell viability, and target gene expression levels.
Based on the screening results, the team combined the most promising PBAE polymers with mRNAs encoding reporter genes, such as GFP and firefly luciferase (fLuc), to evaluate transfection efficiency. For in vivo studies, they added a polyethylene glycol (PEG)-lipid component to improve the stability and circulation time in the bloodstream and reduce the potential induction of immune reactions.
The resulting nanoparticles were characterised for their size and surface charge, with most formulations falling in the range of 200–300 nm in diameter and exhibiting a positive zeta potential, which indicates their ability to bind negatively charged surfaces (e.g., cell membranes) and is favourable for cellular uptake.
PBAE nanoparticles achieve high transfection rates in human cells
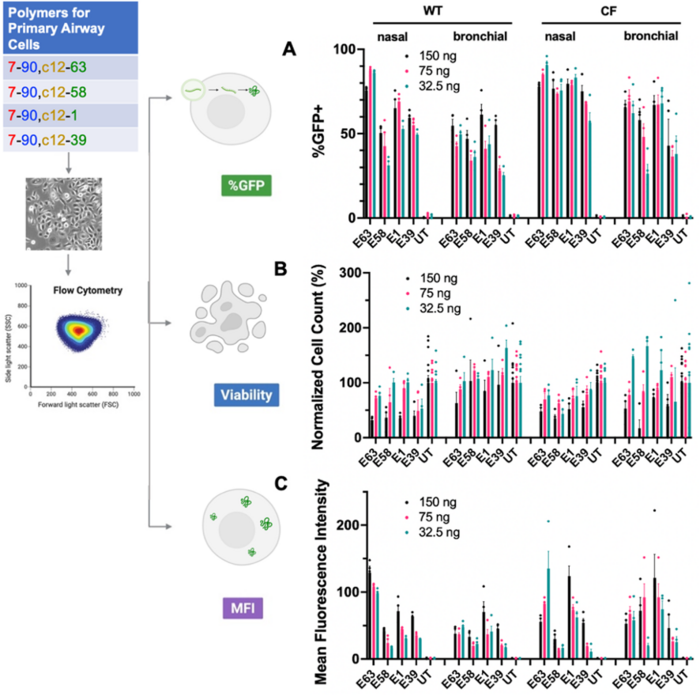
The team tested the nanoparticles on various human airway cell types, including immortalised bronchial epithelial cells from patients with CF and primary nasal and bronchial epithelial cells from healthy donors and patients with CF. Transfection efficiency was assessed using flow cytometry to quantify GFP expression, cell viability using live/dead staining, and gene expression levels using mean fluorescence intensity measurements.
The optimised PBAE nanoparticles, particularly those with the E63 end cap (PBAE-E63), achieved high transfection efficiency in both immortalised and primary human airway epithelial cells. Transfection rates of up to 90% were achieved in primary human nasal and bronchial epithelial cells from both healthy individuals and patients with CF (Figure 1). Cell viability after treatment with PBAE nanoparticles remained high (typically between 75% and 100%) across polymer types and doses, except when a high dose (150 ng) of the nanoparticles was used.
According to Kavanagh, this level of efficiency is unprecedented for non-viral gene delivery systems. Regarding the potential clinical implications of this high efficiency, Kavanagh noted: »When patients with CF can gain even 10% of wild-type recovery, they have a significant improvement in their disease, so they do not have to be completely cured of their disease to see improvements in symptoms and quality of life.«
She added, »If we were to look at, for example, delivering a CRISPR-Cas9 technology and we were able to get 5%–10% of wild-type recovery, that would make a very big clinical impact on that patient.«
PBAE nanoparticles successfully deliver genetic material to the lungs of mice
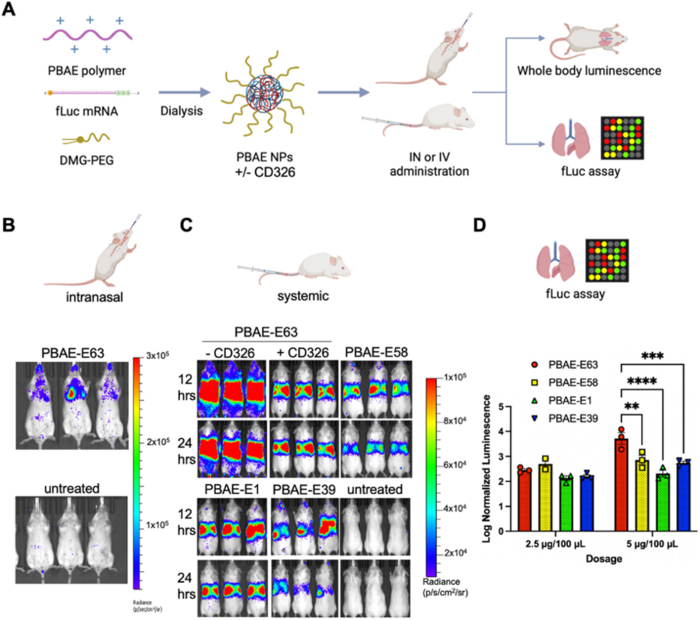
The researchers administered the nanoparticles to mice via intravenous injection to assess their ability to deliver genetic material in vivo. They mixed PBAE polymers with fLuc mRNA and PEG-lipid DMG-PEG2k and administered them intranasally and intravenously. After administration (12 and 24 h), bioluminescence imaging was used to track the distribution and expression of the luciferase. The team explored various factors that could affect delivery efficiency, including the number of doses and addition of a targeting ligand (anti-CD326).
When administered intravenously, nanoparticles showed a strong preference for lung tissue. Bioluminescence imaging revealed high luciferase levels in the lungs and minimal expression in other organs, demonstrating the ability of the system to overcome the challenge of targeted delivery to the lungs via systemic administration (Figure 2).
»Our guess from previous research is that the strong preference of these nanoparticles for lung tissue has something to do with the protein coating,« noted Kavanagh. She explained, »As the nanoparticles enter the bloodstream, they are coated with several proteins, and this protein coating targets them to certain cells.« Kavanagh also emphasised the importance of a systemic approach for the treatment of CF, saying that »CF is ultimately a whole-body disease, and that is why we also wanted to approach it systemically.«
Repeated systemic administration of PBAE nanoparticles leads to DNA editing in lung cells of mice
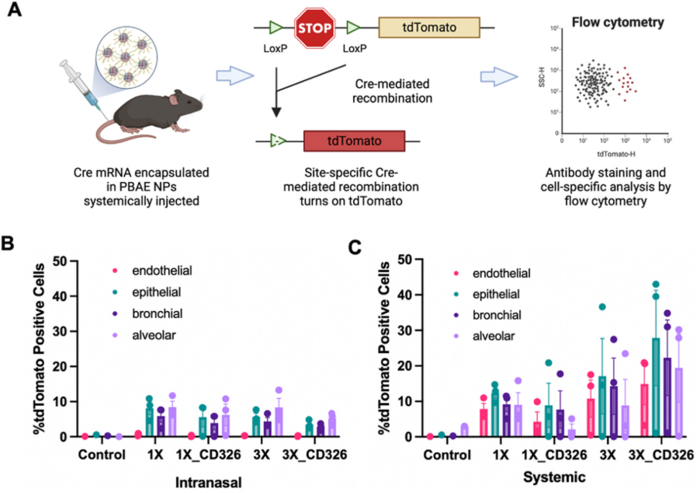
The team employed a genetic mouse model (Ai9) to assess cell-specific targeting and gene-editing efficiency in lung tissues. They injected mice with DMG-PEG-coated PBAE-E63 nanoparticles encapsulating Cre mRNA, and 3 days after administration, they evaluated single-cell tdTomato levels, the expression of which was activated by Cre-mediated recombination. The researchers demonstrated that their nanoparticles could target and transfect various lung cell types, including endothelial, epithelial, bronchial, and alveolar cells (Figure 3).
»For the alveolar cell populations, there are several genetic surfactant disorders that have no treatment currently, and this could potentially also be a therapeutic intervention for those patients,« Kavanagh pointed out.
Approximately 5%–10% of lung cells expressed tdTomato 3 days after intranasal administration of nanoparticles, whereas the percentage of edited lung cells was higher (up to 40%) 3 days after systemic administration of nanoparticles.
Furthermore, multiple administrations of nanoparticles increased the transfection rates across all lung cell types evaluated. After three doses, gene editing was observed in up to 50% of certain lung cell populations.
»It seems that saturation of the cell population was possible, and the golden dosage was three doses, which provided the most transfections with the least number of injections,« Kavanagh stated.
Notably, addition of a targeting ligand (anti-CD326) did not significantly increase DNA editing in the lung cells of mice. Kavanagh explained this observation, saying that »the targeting of CD326 is meant to target epithelial cells, and when you deliver systemically, the first cells to be reached are the endothelial cell layer.« She added that this cellular barrier must be transversed to reach the epithelial cells. Kavanagh also said that having a simpler delivery system with fewer ingredients makes the manufacturing process faster and less costly.
Repeated systemic administration of PBAE nanoparticles is safe
The team performed multiple administrations (up to nine times) of nanoparticles to evaluate potential toxicity through body weight monitoring, blood tests, and histological examination of lung tissues. The nanoparticles showed no significant toxicity, even after multiple injections. The researchers observed no adverse effects on body weight, liver and kidney function, or lung histology.
Kavanagh commented on the potential for repeated long-term treatments: »I think PBAE nanoparticles are well tolerated because they are biodegradable, so they are excreted from the body within 24 hours. They also have a PEG attached to the nanoparticles, which helps reduce their immunogenicity.« She also emphasised that the ability to redose is a key advantage of these nanoparticles compared to AAV delivery methods, as AAVs trigger immune responses that preclude the ability to redose.
Looking ahead
Researchers are currently exploring the use of this delivery system in CRISPR-based therapies. Kavanagh said, »We’re specifically working on encapsulating adenine base editors, coupling with an optimised single-guide RNA to correct a rare CF variant.« She revealed that this approach provided recovery of protein function to a clinically significant level in both immortalised and primary cells carrying this CF variant. »We plan to move into a mouse disease model to see if this targeting of the lungs remains true across the species,« she added.
Regarding large-scale production and clinical translation, Kavanagh expressed optimism: »One of the advantages of using these PBAEs is they’re very easy to manufacture through a two-step chemical reaction, so it’s very cost-effective compared to AAVs.« She added that they have been able to scale up for large animal studies, so she does not foresee significant challenges with large-scale production for their clinical study.
Link to the original article in Biomaterials:
Christos Evangelou, PhD, is a freelance medical writer and science communications consultant.
Tags
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







