Gene-Editing Clinical Trial Update
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
FT538: An Off-The-Shelf Natural Killer Cell Therapy for Acute Myeloid Leukaemia and Multiple Myeloma
FT538 is a universal, off-the-shelf natural killer (NK) cell cancer immunotherapy that is being developed by Californian biopharmaceutical company Fate Therapeutics.
The novel therapy is being tested in two separate treatment regimens in a Phase 1 dose-finding study; either as monotherapy for acute myeloid leukaemia (AML) or as combination therapy with monoclonal antibodies for multiple myeloma (MM).
The study, which aims to enrol up to 105 adult participants, will consist of a dose-escalation stage and an expansion stage where participants will be enrolled into indication-specific cohorts. The dose-escalation stage aims to determine the maximum tolerated dose (MTD) of up to three doses of FT538 at set intervals during the treatment period for the two separate regimens mentioned above. During dose-expansion, the safety, efficacy, and pharmacokinetics of FT538 will be further characterised in all regimens.
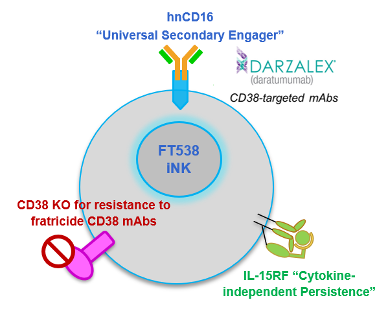
Boosting Innate Immunity to Cancer With Edited Natural Killer Cells
FT538 is edited with CRISPR-Cas technology to bear 3 functional modifications compared to wild-type NK cells. These modifications are designed to boost innate immunity to tumour cells.
Firstly, FT538 cells are derived from a proprietary clonal master iPSC line that is engineered to not express CD38. CD38 is a glycoprotein that is often expressed on myeloma cells, and CD38-targeting monoclonal antibodies (mAbs), such as the FDA-approved daratumumab, are effective in treating myeloma through multiple mechanisms, including antibody-dependent cellular cytotoxicity (ADCC), which causes targeted cells to apoptose. However, because activated wild-type NK cells also express CD38 on their surfaces, daratumumab may induce NK cell depletion, compromising treatment effectiveness.
At last year's American Society for Hematology annual meeting, Fate Therapeutics shared pre-clinical data demonstrating that a lack of CD38 expression resulted in greater long-term daratumumab-mediated ADCC in MM.
FT538 is also engineered to express an IL-15 receptor alpha fusion protein and a high-affinity non-cleavable CD16 to further boost ADCC. These features are intended to further boost ADCC and enhance NK cell persistence, thereby improving outcomes for MM patients.
The overall strategy to boost NK cells is based on clinically-observed deficiencies in NK cell activity during early-stage myeloma, which may be a result of the cancer itself and/or cancer therapy, which become more severe as the disease progresses.
In May of this year, Fate Therapeutics announced that it had received IND clearance for FT538 from the FDA, and details of this Phase 1 study were announced last month.
FT819: An Off-The-Shelf CAR T-Cell Therapy for B-Cell Malignancies
In a recent IND update, we shared that Fate Therapeutics was developing FT819, an off-the-shelf CAR T-cell cancer immunotherapy candidate for the treatment of acute lymphoblastic leukaemia (ALL).
Since then, Fate Therapeutics has initiated a Phase I dose-finding study for FT819 as monotherapy and in combination with IL-2 in participants with relapsed/refractory B-cell malignancies including B cell lymphoma, chronic lymphocytic leukaemia (CLL) and precursor B-cell ALL (B-ALL). The study comprises a dose-escalation stage and an expansion stage where participants will be enrolled into indication-specific cohorts. The trial aims to enrol up to 297 adult participants with an estimated primary completion date in December 2022.
FT819 is CRISPR-engineered to express a novel 1XX CAR that targets the CD19 B cell-specific cell-surface antigen that is expressed in all B cell lineage cancers. The company presented encouraging in vivo pre-clinical data at last year’s American Society of Hemoatology Meeting, and obtained IND clearance from the FDA in July of this year, allowing it to proceed with the clinical development of FT819 for CD19+ cancers.
FT819 is the first-ever CAR T-cell therapy to be derived from a clonal master iPSC line engineered with Fate Therapeutic's proprietary iPSC platform, and it has several first-of-kind features that are designed to improve the safety and efficacy of CAR T-cell therapy.
CB-010: An Off-The-Shelf Allogeneic CAR-T Cell Therapy for B Cell Non-Hodgkin Lymphoma
Caribou Biosciences, a genome-editing company based in Berkeley, California, is developing CB-010 as an off-the-shelf allogeneic anti-CD19 CAR-T cell therapy for relapsed/refractory B cell non-Hodgkin lymphoma.
CB-010 is Caribou’s most advanced cell therapy candidate and is derived from healthy donor T cells that are genome-edited using Caribou’s next-generation CRISPR-Cas technology that is based on CRISPR hybrid RNA-DNA (chRDNAs). Using CRISPR, the PD-1 gene is deleted from CAR-T cells. This gene encodes the PD-1 protein which functions as a safety switch on T cells that cancer cells turn on to protect themselves from T cell-mediated immune responses.
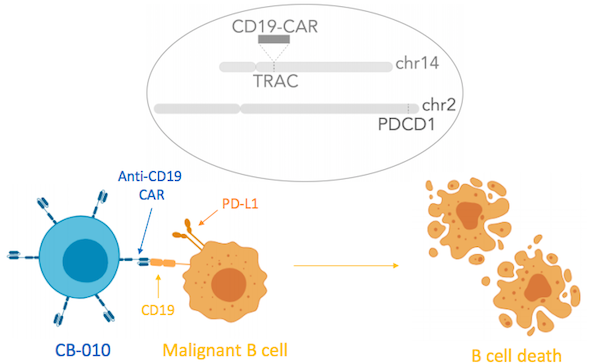
Caribou Biosciences announced IND clearance for CB-010 in September 2020, in which the company also revealed that PD-1 deletion promoted an increase in the durability of anti-tumour activity in pre-clinical studies. Like many other gene-edited CAR T therapies, the endogenous T cell receptor is also deleted in CB-010 in order to prevent graft-versus-host disease and to ensure insertion of the CAR into endogenous T cell receptor (TCR) locus.
The ANTLER Trial
CB-010 has now entered clinical development, with a Phase 1 trial called ‘ANTLER’ to evaluate the safety, emerging efficacy, pharmacokinetics and immunogenicity of CB-010 in adults with relapsed/refractory B cell non-Hodgkin lymphoma after prior lymphodepletion therapy with cyclophosphamide and fludarabine. The trial, which aims to enrol 50 adult participants, is expected to begin this month with an anticipated primary completion date in August 2025.
CB-010 is the first allogeneic CAR-T cell therapy with PD-1 deleted by CRISPR genome editing to be cleared for Phase 1 clinical trials.
For a complete overview of current gene editing clinical trials, check out CRISPR Medicine News' Clinical Trials Database.
Tags
Articlein vivoAcute Lymphoblastic Leukemia, ALLAcute Myeloid Leukemia, AMLB-cell Malignancy, NHLNon-Hodgkin Lymphoma, NHLT-Cell LymphomaCAR-TCRISPR-CasCas9Trials
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







