Highlight: Self-limiting gene drive suppresses malaria mosquitoes
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
Malaria claimed over 600,000 lives in 2022, with Anopheles gambiae serving as one of the most efficient vectors in sub-Saharan Africa, where approximately 96% of malaria deaths occur. The emergence of insecticide resistance threatens progress in disease control, prompting the development of genetic strategies to address it. CRISPR-homing gene drives have emerged as the most studied self-sustaining approaches, whilst various self-limiting methods that require repeated releases continue to be explored.
“In both release cages, populations collapsed after eight consecutive releases of MDFS males”Strampelli et al.
The research team developed a system, termed Male-Drive Female-Sterile (MDFS), that exploits CRISPR-Cas9 to simultaneously perform two distinct functions (see Figure 1). The genetic construct contains an eCFP fluorescent marker, a Cas9 endonuclease under the control of the germline vasa2 promoter, and a guide RNA targeting the female-specific exon 5 of the doublesex gene. The construct was integrated into the doublesex locus at the intron 4–exon 5 boundary using recombinase-mediated cassette exchange.
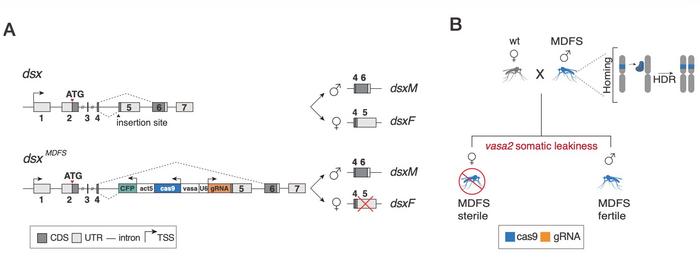
The vasa2 promoter proved critical to enabling the dual functionality of CRISPR-Cas9 in the system. This promoter drives Cas9 expression in the germline of both sexes but also exhibits somatic leakiness. In males, germline Cas9 expression enables the characteristic homing mechanism. The Cas9 endonuclease, guided by the gRNA, recognises and cleaves the wild-type doublesex allele on the homologous chromosome, which is then converted into an MDFS allele through homology-directed repair. This CRISPR-mediated homing allows the construct to be transmitted to offspring at super-Mendelian rates, far exceeding 50% Mendelian inheritance rates. In females, somatic leakiness causes Cas9 to be expressed throughout developing tissues, where CRISPR-Cas9 disrupts the doublesex gene in somatic cells, converting them to homozygosity for a null allele and resulting in dominant female sterility.
MDFS females displayed a striking intersex phenotype resulting from CRISPR-Cas9 activity in their somatic tissues. All examined females developed male claspers, exhibited male-like head morphology, and had rudimentary ovaries. When given the opportunity to blood-feed, MDFS females were unable to do so, and crosses with wild-type males produced no progeny. Pooled amplicon sequencing provided direct evidence of CRISPR-Cas9 cleavage activity throughout development, revealing extensive cutting of the wild-type doublesex allele that increased from 32.4% at the first larval stage to an average of 85.3% in adult females. The CRISPR-mediated cleavage produced over 20 different repair variants, predominantly small insertions and deletions centred around the cut site, resulting from error-prone non-homologous end-joining repair.
The fertility of MDFS males proved comparable to wild-type controls, indicating that germline CRISPR-Cas9 activity does not impose fitness costs. Males carrying only the MDFS allele produced an average of 68.6 larvae per female, not significantly different from the wild-type average of 77.6 larvae. The inheritance of the MDFS allele was remarkably high at 99.5% to 99.7%, confirming highly efficient CRISPR-mediated homing. The guide RNA successfully directed Cas9 to cleave the target site with exceptional precision, enabling near-complete conversion of wild-type alleles through homology-directed repair. When combined with an X-shredder component (using I-PpoI endonuclease), males produced the expected male bias of 93.6% in their progeny.
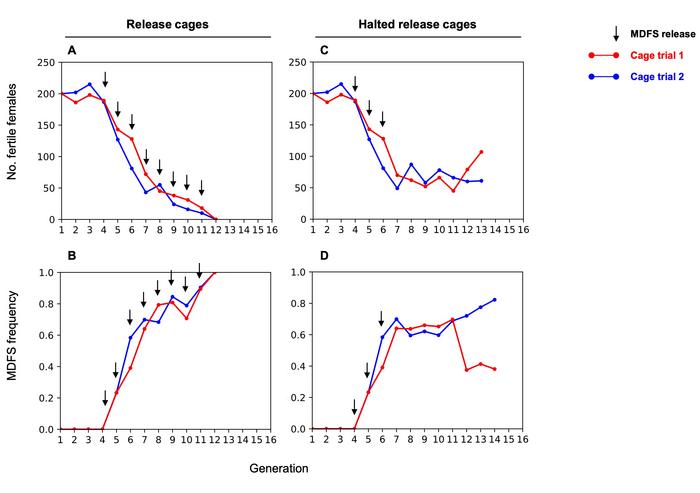
Cage trials provided compelling evidence for the system's efficacy (see Figure 2). Two replicate cages initially seeded with 400 wild-type individuals received releases of 100 MDFS males at each generation. Following the third release, each cage was duplicated: one continued receiving releases whilst the sister cage received no further releases. In both release cages, populations collapsed after eight consecutive releases, at which point 100% of individuals carried the MDFS allele and all females were sterile – a direct result of CRISPR-Cas9 activity.
»In both release cages, populations collapsed after eight consecutive releases of MDFS males. At that point, 100% of the cage trial populations carried the MDFS allele, and all females were intersex and thus unable to reproduce,« the authors note in the paper. Conversely, the halted-release cages maintained stable population trends for over eight generations, confirming the self-limiting nature of the approach.
During cage trials, researchers detected rare females carrying an 11-base pair deletion – a dominant negative mutation in the doublesex locus likely arising from CRISPR-Cas9 activity in male germlines. Importantly, even these CRISPR-generated resistance alleles maintained female sterility, potentially limiting the evolution of functional resistance.
Mathematical modelling predicted that the CRISPR-based MDFS would outperform other self-limiting strategies. To achieve 99% population suppression within 36 generations, MDFS would require 13-fold lower releases than the optimal sterile insect technique, 4.7-fold lower than the optimal RIDL, and 4.3-fold lower than an X-shredder. When combined with an X-shredder, release rates could be reduced by over 60% compared to MDFS alone.
The research demonstrates that CRISPR-Cas9 can be leveraged in innovative ways beyond simple gene disruption or standard homing gene drives. By exploiting both germline activity for super-Mendelian inheritance and somatic leakiness for dominant female sterility, the MDFS system achieves efficient population suppression whilst maintaining self-limiting characteristics. The approach addresses concerns about uncontrolled spread associated with self-sustaining CRISPR gene drives whilst reducing the release burden of purely self-limiting technologies.
The study was led by Anna Strampelli and Federica Bernardini from Imperial College London, UK. It was published on 27 October 2025 in Nature Communications.
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleCMN HighlightsNewsMalariaInfectious diseases - otherGene driveSterile Insect Technique (SIT)Cas9
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







