Hydrogel enhances CRISPR-mediated tumour targeting
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
The work is grounded in the broader limitations of systemic CRISPR-Cas9 delivery, which often suffers from short half-life, immune clearance, and non-specific distribution. Traditional delivery methods, such as viral vectors, are hampered by immunogenicity and payload limitations. At the same time, non-viral carriers like liposomes and nanoparticles have lower delivery efficiencies and lack localisation to tumour sites.
“The strategy of combining YB-1 gene editing and intratumoral injection enhanced the therapeutic effect of doxorubicin while reducing side effects”Li et al.
In contrast, in situ hydrogels offer sustained release, biocompatibility, and tumour-specific retention. The authors specifically targeted Y-box binding protein 1 (YB-1), a known driver of melanoma proliferation and chemoresistance, using Cas9 RNP complexes. Co-delivery with the chemotherapeutic doxorubicin was intended to synergise the effects of gene editing and cytotoxicity.
“The strategy of combining YB-1 gene editing and intratumoral injection enhanced the therapeutic effect of doxorubicin while reducing side effects,” the authors note in their paper.
Three hydrogels were engineered with distinct surface charges—neutral (Nuh), negative (Ngh), and positive (Psh)—using poloxamer polymers. The Psh hydrogel, modified with synthetic polyamines, showed the highest encapsulation efficiency (91%) and slower release kinetics. In vitro, Psh achieved greater cellular uptake and editing efficiency than other formulations or standard transfection reagents. The Psh@DOX@RNP hydrogel induced apoptosis and significantly inhibited melanoma cell proliferation, achieving a YB-1 indel rate of 53% in B16F10 cells. Similar performance was observed in a GFP-reporter assay using human cells.
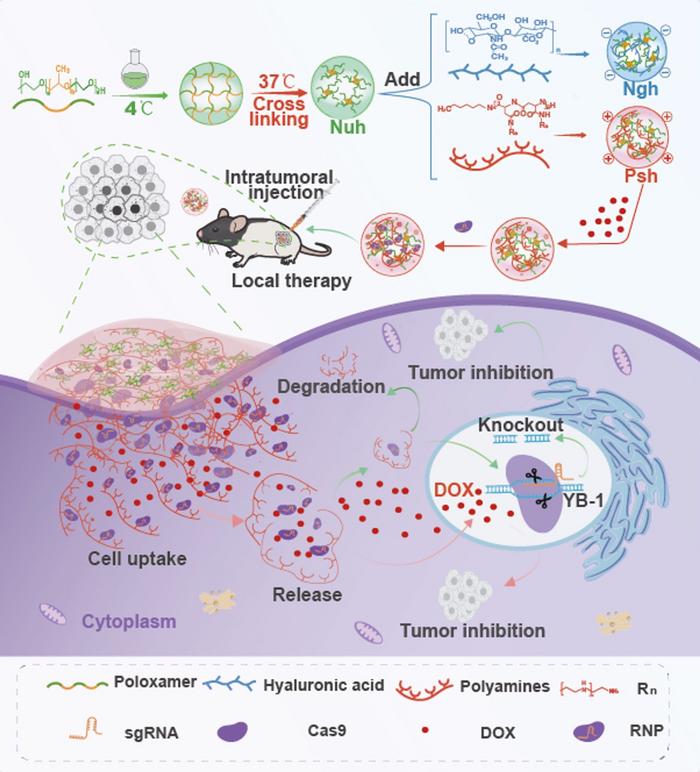
In vivo, intratumoral injection of Psh-based hydrogels into a murine melanoma model led to enhanced tumour retention and delayed release of both DOX and CRISPR-Cas9 RNP. Fluorescence imaging over eight days confirmed that Psh maintained significantly higher levels of both components in tumour tissues compared to neutral or negatively charged formulations.
Tumour growth inhibition exceeded 98% in the Psh@DOX@RNP group, with substantial histological evidence of cell death, reduced proliferation (as measured by Ki-67 staining), and increased apoptosis (TUNEL assay). Importantly, no detectable off-target editing was observed in heart, liver, spleen, lung, or kidney tissues, and complete blood counts and histopathology confirmed systemic safety.
“Our gel formulation and delivery strategy show significant benefits in blocking melanoma and could offer novel approaches for other surface tumours of the body”Li et al.
The authors conclude that their polyamine-modified hydrogel facilitates efficient co-delivery of gene-editing agents and chemotherapeutics, enhancing tumour localisation and gene disruption while minimising systemic exposure. The combination of local administration, sustained release, and positive surface charge appears to be key to achieving efficient genome editing and therapeutic synergy.
This approach holds promise not only for melanoma but potentially for other solid tumours accessible to local injection. However, the authors acknowledge that further studies are needed to confirm safety in human models and evaluate efficacy in metastatic settings:
“Our gel formulation and delivery strategy show significant benefits in blocking melanoma and could offer novel approaches for other surface tumours of the body.”
The study was conducted by Meng Li, Songli Zhou, Suqin Zhang and colleagues at Hubei University and Tongji Hospital, Huazhong University of Science & Technology. It was published in the Journal of Nanobiotechnology on 13 June 2025.
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleBreaking newsCMN BriefsNewsDeliveryNon-viralRibonucleoprotein (RNP)Cancer
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







