IND Update: Gene-Edited CAR Natural Killer Cell Therapy for Solid Tumours
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
Fate Therapeutics recently announced that the FDA had approved its IND application for FT536 to enter a Phase 1 clinical trial for advanced solid tumours.
FT536 is designed as a first-in-class, off-the-shelf, chimeric antigen receptor (CAR) natural killer (NK) cell product candidate. It is derived from a clonal master induced pluripotent stem cell line that undergoes multiple gene-editing steps. These include the addition of a novel CAR that targets MICA and MICB, immune-activating stress proteins that are abundantly expressed by many solid and haematopoeitic tumour types.
According to a company press release, Fate Therapeutics plans to initiate a clinical study for FT536 as mono- and combination therapy along with tumour-targeting monoclonal antibody therapy for the treatment of multiple solid tumour indications.
Eligible cancer types include advanced non-small cell lung cancer, colorectal cancer, head and neck cancer, gastric cancer, breast cancer, ovarian cancer, and pancreatic cancer.
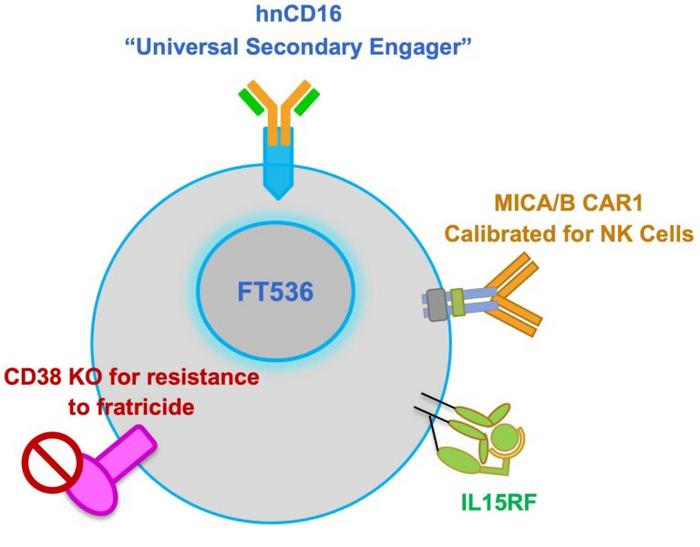
FT536 mechanism of action
MICA and MICB are expressed by many cancer cell types in response to cellular stress and constitute components of the natural anti-tumour immune response.
Expression of MICA and MICB tags the cancer cells for removal, whereby NK cells and CD8+ T cells can recognise and bind to the α1 and α2 domains of MICA and MICB, thus activating a potent cytotoxic response.
It is recognised that cancer cells can escape this immune response through proteolytic shedding of the α1 and α2 domains of MICA and MICB. FT536’s proprietary CAR targets the α3 domain of MICA and MICB and has been demonstrated to overcome shedding and restore NK and T cell-mediated tumour immunity.
FT536 is the first cell-based therapeutic candidate to target MICA and MICB. The therapeutic strategy is based on research findings published in Science in 2018 that validate the α3 domain as a therapeutic target. This work, which was led by Dr. Kai W. Wucherpfennig at the Dana-Farber Cancer Institute, demonstrated that anti-α3 antibodies led to inhibiton of tumour growth in multiple animal solid cancer models. Fate Therapeutic’s FT536 programme is supported by an exclusive license from the Dana-Farber Cancer Institute to intellectual property covering novel antibody fragments binding MICA/B for iPSC-derived cellular therapeutics.
Besides the unique α3-specific CAR, FT536 also harbours a so-called ‘Universal Secondary Engager’, which is a modified CD16 receptor that is engineered to prevent its down-regulation and to enhance its binding to tumour-targeting antibodies. Additional edits include the addition of an IL-15 receptor fusion that augments NK cell activity as well as knockout of the CD38 gene, which promotes cell persistence and function in the hostile tumour microenvironment. Details about the gene-editing strategy employed have not been disclosed.
The FT536 trial
So far, the company has announced plans to initiate a six-arm multi-center Phase 1 trial to evaluate a multi-dose, multi-cycle treatment schedule with FT536 as monotherapy or as combination therapy along with any of five cancer-specific monoclonal antibody therapy for patients with advanced solid tumours. The off-the-shelf treatment regimen is designed to be administered in the outpatient setting.
We will continue to provide updates about FT536 and the upcoming clinical trial as further details emerge.
Tags
ArticleNewsin vivoCancerSolid Tumor AdultSolid TumoursCRISPR-CasCas9Fate Therapeutics, Inc.TrialsIND - Investigational New Drug
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







