Latest Gene Editing Clinical Trials for Cancer
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
Here are the latest additions to our overview of gene editing trials for cancer. Two are for lymphomas and four are for leukaemia. Each trial deploys either CRISPR-Cas9-, TALEN- or meganuclease-mediated gene editing technology to engineer chimeric antigen receptor (CAR)-T cell therapies.
CRISPR-Cas9-Edited T Cell Therapy for B-Cell Acute Lymphoblastic Leukaemia (NCT04557436)
First up is a Phase 1 open-label trial for B-cell acute lymphoblastic leukaemia (B-ALL). The paediatric trial, which is sponsored by Great Ormond Street Hospital for Children NHS Foundation Trust (UK) in collaboration with University College London, will evaluate safety and efficacy of an investigational T cell therapy (TT52CAR19) in children aged 6 months to 18 years with relapsed or refractory B-ALL.
TT52CAR19T is an engineered T cell therapy that is generated ex vivo using a lentivirally-delivered anti-CD19 CAR, dual guide sgRNA cassettes targeting the T cell receptor (TCR) alpha chain (TRAC) and CD52 loci, and Cas9 mRNA delivered via electroporation. CD19 is a B cell-specific cell surface antigen expressed in all B cell lineage malignancies. TRAC disruption mitigates the risk of graft vs host disease, while CD52 deletion renders TT52CAR19T resistant to the anti-CD52 monoclonal antibody alemtuzumab, which is used during lymphodepletion.
It is anticipated that 10 UK-based patients will be enrolled in this trial, which will evaluate the safety of TT52CAR19 and determine whether it can mediate molecular remission by day 28 of treatment and thereby allow patients to proceed to allogeneic haematopoietic stem cell transplantation (allo-SCT), which remains part of the backbone therapy for these patients. LTT52CAR19 will be administered to each patient once and they will be depleted by standard pre-transplant conditioning 28 days later. Patients will be followed up for 3 years after the trial.
Professor Waseem Qasim of University College London and Great Ormond Street Institute of Child Health is involved in the study, and we recently interviewed him about the role of gene editing for CAR T-cell therapeutic development.
TALEN-Edited CAR T-Cells for B-Cell Acute Lymphoblastic Leukaemia (NCT02746952 and NCT02808442)
Institut De Recherches Internationales Servier, an international research company and part of the Servier Group, headquartered in France, has sponsored two Phase 1 trials for B-cell acute lymphoblastic leukaemia (B-ALL) in adult and paediatric participants at multiple international study sites. Both trials are open-label and assess the TALEN-edited CAR T-cell therapy UCART19. UCART19 is an allogeneic, frozen ‘off-the-shelf’ T lymphocyte therapy that is developed using TALEN technology, as depicted in Figure 1.
UCART19 T cells express an anti-CD19 CAR that mediates targeting of CD19+ cells, as well as the RQR8 transgene. RQR8 is engineered to contain epitopes from CD34 and CD20, which allows tracking of the UCART19 cells with a clinically approved anti-CD34 antibody. Using TALEN technology, the T-cell receptor (TCR) alpha chain and CD52 genes are deleted from the CAR19 T cells. Upon infusion, UCART19 T cells specifically target and bind to CD19-expressing tumour cells, causing them to lyse. The knockout of the TCR alpha gene eliminates TCR expression and is intended to abrogate the potential induction of graft-versus-host disease (GvHD) by the donor T cells. As an inbuilt safety feature, the CD20 portion of the CAR permits selective depletion of the UCART19 cells when the anti-CD20 monoclonal antibody rituximab is administered, in the case of unacceptable adverse effects of UCART19.
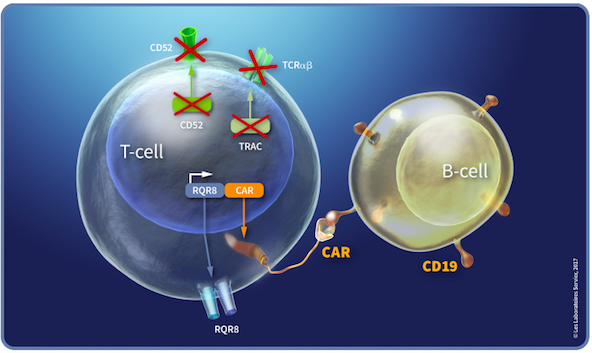
UCART19 cells are faster to produce and provide ‘off-the-shelf’ CAR T-cells when compared to currently marketed autologous CAR T-cell therapies, which use patients’ own cells and are produced on a bespoke basis.
The first trial is a 2-part safety study of UCART19 in up to 40 adult patients with B-ALL. The study, known as CALM, comprises a dose escalation followed by a safety dose expansion. During dose escalation, one set of patients is treated with single infusions of ascending doses of UCART19 in order to identify the maximum tolerated dose (MTD), the recommended dose and the lympodepletion regimen. The safety expansion study examines the safety and tolerability of the MTD in an additional cohort of participants. Preliminary results from the CALM trial were shared byCellectis(which developed and then licensed UCART19 toServierfor clinical development) earlier this year, which revealed promising safety and efficacy data as well as expansion of UCART19 cells in treated patients.
The second UCART19 trial is also open-label is currently recruiting 18 paediatric patients with B-ALL to evaluate the safety and feasibility of UCART19 to induce molecular remission within 28 days of the first treatment. This study began in 2016 and preliminary safety results from 5 patients treated in this trial were published in the journal Blood in 2017. The final data collection for the primary endpoint will take place in 2021.
Meganuclease-Edited CAR T-Cell Therapies
Meganucleases are a group of naturally occurring and highly specific restriction enzymes that were first discovered and recognised to have gene-editing potential in the 1990s. However, in spite of their high specificity and low cell toxicity, their natural diversity is limited making it difficult to realise their gene-editing potential for broad therapeutic purposes. In recent years, companies have developed platforms to engineer tailor-made meganucleases with specificity for desired sequences through modifications to the enzyme’s recognition site or by mixing and matching domains from distinct meganucleases into recombinant versions with the sought after properties.
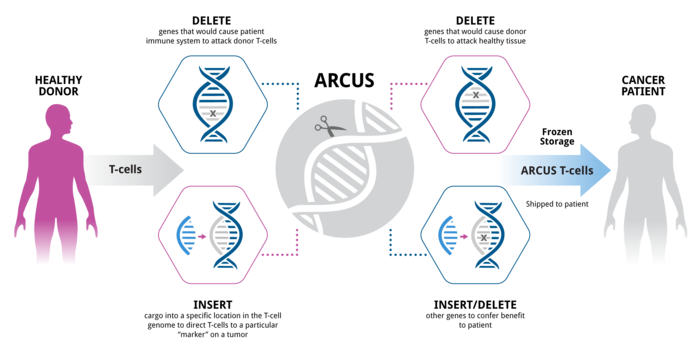
Precision BioSciences (US) has developed a next-generation meganuclease platform called “ARCUS” that can produce nucleases with customised activity and specificity. The ARCUS nucleases are capable of distinguishing target sites that differ by only 1 base pair. Figure 2 illustrates the workflow of the ARCUS platform.
Precision BioSciences are currently sponsoring three clinical trials that deploy tailor-made meganucleases to engineer CAR-T cells for treatment of multiple myeloma (MM), B-cell Acute lymphoblastic leukaemia (ALL) and Non-Hodgkin Lymphoma (NHL) or Chronic Lymphocytic Leukaemia (CLL) or Small Lymphocytic Lymphoma (SLL).
PBCAR0191 for Advanced B-Cell Precursor Acute Lymphoblastic Leukaemia (NCT03666000)
The first study evaluates Precision BioSciences’ lead investigational allogeneic chimeric antigen receptor (CAR T) cell therapy, PBCAR0191. This is an ‘off-the-shelf’ allogeneic CAR T therapy that uses T cells from healthy adult donors. Using ARCUS technology, the T cells are engineered in a single step that inserts the CAR0191 gene at the TCR locus, thus replacing the native TCR and creating a cell product that targets CD19 and which is also expected to prevent GvHD by disruption of the native TCR locus.
Ninety-two patients are enrolled from multiple locations worldwide, and dosing began last year. In December 2019, Precision BioSciences released promising safety and efficacy data at the American Society Hematology. Participants undergo lymphodepletion with chemotherapeutic agents prior to treatment with either a single dose or a split dose of intravenously-infused PBCAR0191, and all patients who receive PBCAR0191 will be followed up for 15 years in a separate long-term follow-up study. PBCAR0191 is being developed in collaboration with Servier, an international pharmaceutical company, and Precision BioSciences announced in August 2020 that it had received fast track disease designation from the U.S. Food and Drug Administration (FDA) for PBCAR0191 for the treatment of advanced B-cell precursor acute lymphoblastic leukaemia (B-ALL).
PBCAR269A for Relapsed or Refractory Multiple Myeloma (NCT04171843)
The second trial is for a meganuclease-edited CAR T-cell therapy called PBCAR269A, for the treatment of relapsed or refractory multiple myeloma (MM) in adults. This is a non-randomised open-label Phase 1/2a trial occurring in multiple sites around the world, which encompasses single dose, dose escalation (to determine the MTD), and dose expansion studies to evaluate the safety and efficacy of PBCAR269A in adult participants.
PBCAR269A is generated in a similar manner to PBCAR0191. Using ARCUS technology, CAR269A replaces the native TCR, creating a cell product that targets the B-cell maturation antigen (BCMA), a cell surface protein universally expressed on malignant plasma cells, which is one of the hallmarks of MM.
Participants will undergo lymphodepletion with chemotherapeutic agents prior to treatment with PBCAR269A and all patients who receive treatment with PBCAR269A will be followed up for 15 years. Precision BioSciences reported that the first participant was dosed in June 2020, and results from the MTD determination are expected in May 2021.
PBCAR20A for Non-Hodgkin Lymphoma (NHL) or Chronic Lymphocytic Leukaemia (CLL) or Small Lymphocytic Lymphoma (SLL). NCT04030195
The third trial is a non-randomised open-label Phase 1/2a trial occurring in multiple sites around the world, which encompasses single dose, dose escalation (to determine the MTD), and dose expansion studies to evaluate the safety and efficacy of PBCAR20A in participants with relapsed/refractory (r/r) Non-Hodgkin Lymphoma (NHL) or r/r Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL).
PBCAR20A is a meganuclease-edited ‘off-the-shelf’ anti-CD20 CAR T therapy, engineered using ARCUS technology. Before initiating treatment with PBCAR20A, participants will undergo lymphodepletion chemotherapy. On day 0 of the treatment period, subjects will receive a single intravenous infusion of PBCAR20A, and all who receive the treatment will be monitored for 28 days post-treatment. All subjects who receive a dose of PBCAR20A will be followed in a separate long-term follow-up (LTFU) study for 15 years after exiting this study.
For a complete overview of current gene editing clinical trials, check out CRISPR Medicine News' Clinical Trials Database.
Tags
ArticleAcute Lymphoblastic Leukemia, ALLMultiple Myeloma, MMNon-Hodgkin Lymphoma, NHLCAR-TCRISPR-CasMeganucleasesTALENsTrialsClinical
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







