Latest Gene editing Clinical Trials
Here is the latest update to our overview of current clinical trials involving gene editing.
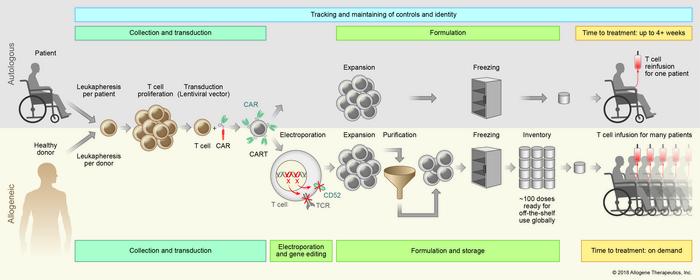
We have added two new trials - both cancer immunotherapy trials. The studies, called ALPHA and UNIVERSAL by Allogene Therapeutics, are investigational 'off-the-shelf' CAR T therapies using T cells harvested from healthy donors.
The gene-editing tool used is TALENs, and the gene editing is done ex vivo. Both trials are phase I safety and efficacy studies in adults, and actively recruiting patients at several sites in the US (up to 54 and 90 patients respectively).
ALPHA - non-Hodgkin's lymphoma
The purpose of the ALPHA study is to assess the safety and efficacy of ALLO-501 anti-CD19 allogeneic CAR T cells in adults with relapsed/refractory large B cell or follicular lymphoma.
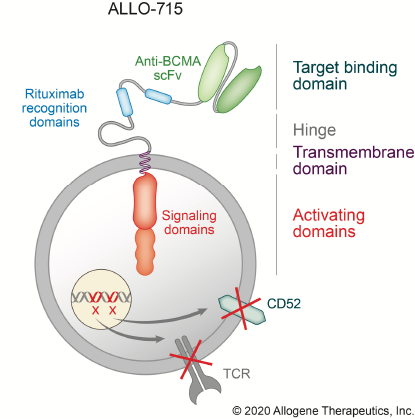
ALLO-501 is an investigational off-the-shelf CAR T Cell therapy using T-cells harvested from healthy donors.
The T cells are isolated in a manufacturing facility, engineered to express synthetic T cell receptors, CARs (chimeric antigen receptors) to recognize and destroy cancer cells, and modified via gene editing to limit autoimmune response when given to a patient.
ALLO-501 specifically recognize CD19, a cell-surface protein expressed on B-cells, including cancerous B-cells and a lentiviral vector is used to add the CAR T receptor.
To edit the genes, TALEN gene-editing tools (Cellectis' technology) are then electroporated ex vivo into the T cells.
Two genes are knocked out - the endogenous T cell receptor to minimize the risk of Graft versus Host Disease and the CD52 gene to render the T cells resistant to anti-CD52 antibody treatment, ALLO-647. The therapy uses an anti-CD52 monoclonal antibody designed to suppress the host immune system and allow the edited CAR T cells to stay engrafted in order to achieve full therapeutic impact.
- Official title: A Single-Arm, Open-Label, Phase 1 Study Evaluating the Safety, Efficacy, and Cellular Kinetics/Pharmacodynamics of ALLO-501, an Anti-CD19 Allogeneic CAR T Cell Therapy in Patients With Relapsed/Refractory Large B-Cell Lymphoma or Follicular Lymphoma.
- Trial ID: NCT03939026.
UNIVERSAL - Multiple myeloma
The purpose of the UNIVERSAL study is to assess the safety and efficacy of ALLO-715 BCMA allogeneic CAR T cells in adults with relapsed or refractory multiple myeloma.
The UNIVERSAL trial is similar to the ALPHA study with ALLO-715 also being an investigational 'off-the-shelf' CAR T Cell therapy using T-cells harvested from healthy donors.
The T cells are engineered ex vivo in the same manner as in the ALPHA study. However, the ALLO-715 the CAR recognizes the BCMA-antigen, a cell-surface protein universally expressed on malignant plasma cells.
The TALEN gene-editing tools knockout the same two genes as the ALPHA study (T cell receptor and the CD52 gene) to minimize the risk of Graft versus Host Disease and render the T cells resistant to ALLO-647 immune repression.
One safety measure is added in the UNIVERSAL trial compared to the ALPHA trial.
The safety of the allogeneic ALLO-715 CAR Ts is further enhanced by incorporating a CD20 mimotope-based intra-CAR off-switch. That safety-switch allows investigators to destroy genetically-modified T-cells in the presence of Rituxan (rituximab) if needed.
- Official title: A Single-Arm, Open-Label, Phase 1 Study of the Safety, Efficacy, and Cellular Kinetics/Pharmacodynamics of ALLO-715 to Evaluate an Anti-BCMA Allogeneic CAR T Cell Therapy in Subjects With Relapsed/Refractory Multiple Myeloma.
- Trial ID: NCT03939026.
Other current trials
With these two studies, the number of current clinical trials involving gene editing is 47 most being therapies for cancer (26) and blood diseases (15).
As a brief overview, the TALEN gene-editing tools are used in eight gene editing trials, that are all cancer treatments with two based in China and six in the US. Of the eight TALEN trials, there is currently two aimed at treating multiple myeloma - the UNIVERSAL study and a study by Cellectis in the US.
Another 18 cancer therapy trials use CRISPR gene editing tools with 10 based in China, 7 in the US and 1 in the UK.
Two of these are investigational treatments for multiple myeloma like the UNIVERSAL study - one by the University of Pennsylvania and one by CRISPR Therapeutics). One of the 18 CRISPR studies is a treatment for non-Hodgkin's lymphoma like the ALPHA study by CRISPR Therapeutics.
Tags
ArticleMultiple Myeloma, MMNon-Hodgkin Lymphoma, NHLCAR-TCas9TALENsSafetyTrialsClinical
CLINICAL TRIALS
Sponsors:
Suzhou Maximum Bio-tech Co., Ltd.
Sponsors:
Zhejiang University







