Minimal Type I-F2 CRISPR-Cas Activates Transcription and Base-Edits DNA
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
CRISPR-Cas systems are adaptive immune systems found in prokaryotes. They are classified into two main classes: Class 1 and Class 2. Class 1 systems (Types I, III, and IV) use multi-subunit complexes for interference, whereas Class 2 systems (Types II, V, and VI) rely on single, multi-domain effector proteins like Cas9 and Cas12.
Type I systems are the most prevalent and are further divided into seven subtypes (I-A to I-G), with the Cascade complex responsible for target recognition and DNA cleavage. The type I-F2 CRISPR-Cas system, part of the Class 1 family, features a compact Cascade complex, which is notable for its smaller size compared to other Type I systems. This feature makes it attractive for applications where cargo size is limited, such as in eukaryotic genome editing.
In this study, the Chinese team developed a compact version of the type I-F2 CRISPR-Cas system derived from Moraxella osloensis, which showed strong potential for transcriptional regulation and base editing in human cells (see Figure 1).
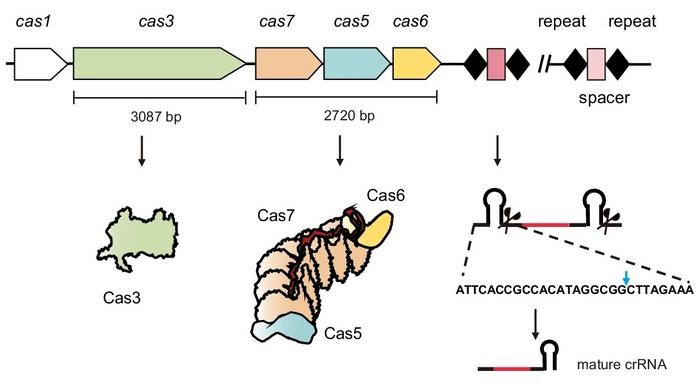
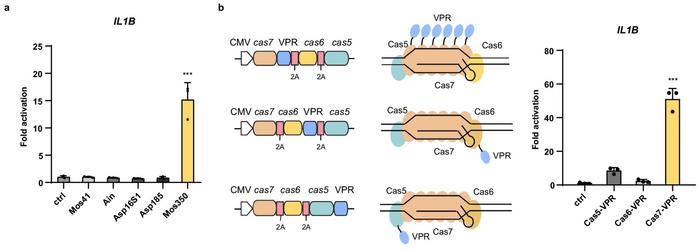
The compact I-F2 system, smaller than the widely used SpCas9, was engineered to perform transcriptional activation by fusing various Cas proteins to an activation domain (VPR). This configuration effectively enhanced the expression of specific genes, such as IL1B and HBB. The researchers achieved significant transcriptional activation, particularly at the IL1B gene, using the Cas7-VPR fusion, which outperformed other configurations (see Figure 2).
They also developed a base editor with a wide (~30 nucleotides) bimodal editing window, showing high efficiency in converting adenines across multiple target sites, with editing rates exceeding 50% in some cases. The engineered type I-F2 system demonstrated greater flexibility and efficiency than existing Cas9 or Cas12 systems for both transcriptional activation and base editing in human cells.
The experimental setup used HEK293T human cells as the primary cell model for testing transcriptional activation and base editing. The CRISPR components were delivered using plasmid transfection, and the human codon-optimised Cascade subunits (Cas5, Cas6, and Cas7) were expressed under a CMV promoter.
This research was led by Hua Xiang from the State Key Laboratory of Microbial Resources, Chinese Academy of Sciences, and it was published in Nature Communications on 23 August 2024.
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







