Neuromuscular Circuits Can Help Evaluate the Functional Outcome of CRISPR in Duchenne Muscular Dystrophy

Mutations in the dystrophin gene (DMD) cause Duchenne muscular dystrophy (DMD), the most common neuromuscular disorder in childhood. DMD patients suffer from progressive skeletal muscle weakness and wasting, resulting in poor quality of life and reduced life expectancy. Unfortunately, the current standard of care can only slow disease progression, and there is still no cure or effective treatment for DMD.
»Progress in finding a cure for neuromuscular disorders has been hampered by the lack of human-relevant pre-clinical models amenable to studying neuromuscular connectivity in healthy and disease conditions and for assessing the efficacy of drug candidates. The aim of our study was to develop such a model by exploiting human pluripotent stem cells, CRISPR-mediated genome editing, bioengineering and light-controllable neuromuscular activity,« says Yung-Yao Lin. He is a geneticist at the Queen Mary University of London and the leader of the team that recently presented such a model in Science Advances.
“Our model will allow CRISPR researchers to evaluate how effective their CRISPR designs are”Yung-Yao Lin
»Since our model allows light-stimulation of motor neuron activity that induces muscle contraction, this will allow CRISPR researchers to evaluate how effective their CRISPR designs are to restore dystrophin protein expression leading to improved neuromuscular connectivity and motor function,« he adds.
Murine motor neurons activate human myofibers
The model is based on a compartmentalised microdevice with neuromuscular circuits. Yung-Yao Lin and his team, in collaboration with Ivo Lieberam at King's College London, populated the central compartment with myofibers from a patient while the two adjoining compartments were plated with murine motor neurons and astrocytes. The motor neurons had previously been genetically engineered to elicit a nerve signal by light, so-called optogenetic activation. After cultivation of the cell-coated microdevice, axons would grow from murine motor neurons to form neuromuscular junctions with human myofibers, and a pulse of light would cause the myofibers to contract.
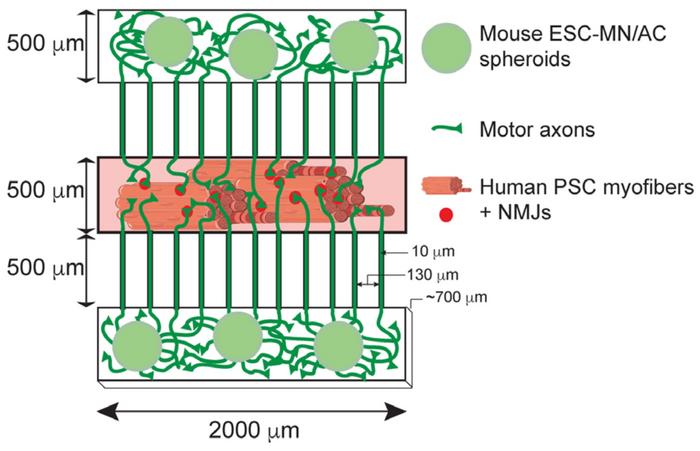
»To mimic the anatomical arrangement of motor neurons and skeletal muscle, the devices were designed to have a central compartment that accommodates the myofibers and two outer compartments for the aggregates of motor neurons and astrocytes. The outer compartments connect to the central compartment via microchannels so that the motor neurons can project their axons to innervate the myofibers,« says Amaia Paredes-Redondo, a former research scientist in Lin's group and the first author of the paper.
A pulse of light shows the effect of CRISPR gene editing
To allow the model to compare the function of mutated and CRISPR-corrected myofibers, fibroblasts from a DMD patient were reprogrammed to obtain pluripotent stem cells (PSCs). Then, before further differentiating them to myotubes and myofibers, some of the PSCs were subjected to CRISPR correction of the disease-causing mutation. In this case, the DMD c.10141C>T mutation was perfectly corrected using homology-directed repair and piggyBac transposon-based selection. In principle, however, any DMD-causing mutation from other patients could be corrected using different CRISPR strategies. Thus, the main thing was to obtain an isogenic pair of patient-specific cells that only differed in having either a mutated or corrected version of DMD.
“We compared the contraction of DMD and CRISPR-corrected myofibers and found that DMD had significantly compromised myofiber contraction”Amaia Paredes-Redondo
»Previous studies had shown that DMD patient-derived skeletal muscle cells recapitulated some pathological features of the disease. However, the observed cellular phenotypes varied from patient to patient because of differences in individual genetic backgrounds. Therefore, it is crucial to generate appropriate isogenic controls for analysis. In our study, we precisely corrected the dystrophin gene mutation in pluripotent stem cells derived from a DMD patient using CRISPR-mediated genome editing,« explains Amaia Paredes-Redondo and continues:
»We used optogenetic stimulation to activate light-responsive motor neurons. Upon specific light activation, the motor axons induced a rapid contraction of the myofibers. This proved that neuromuscular circuits in the compartmentalised microdevices were functional. Then, we compared the contraction of DMD and CRISPR-corrected myofibers and found that DMD had significantly compromised myofiber contraction.«
Contraction velocity is used as a measure of functionality
The observation was based on establishing the velocity of myofiber contraction using particle image velocimetry, which measures the displacement of blocks of pixels between two consecutive images, frame by frame. This was visualised as velocity vectors with different sizes proportional to the local displacement and could be quantitated as contraction velocity. Results revealed a significant effect of CRISPR correction of the mutated DMD gene. Myofibers with the mutated gene had a maximum contraction velocity of ~5 µm/s, tripling to ~15 µm/s in corrected but otherwise isogenic myofibers.
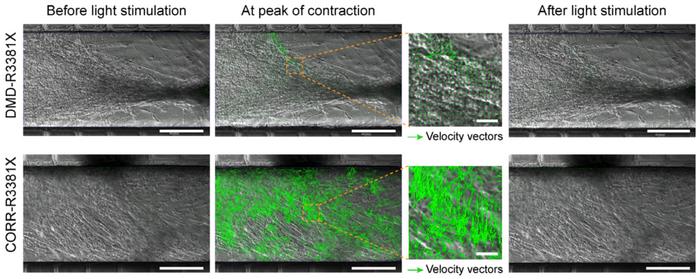
»In our micro-physiological model of neuromuscular circuits, the contraction velocity of myofibers reflect the motor function. As we have shown in our paper, inhibition of TGFβ signalling restored motor function in DMD neuromuscular circuits. Thus, our micro-physiological model can be used to de-risk and prioritise potential therapeutics at the pre-clinical stage of the drug development process,« says Yung-Yao Lin.
The model is a drug screening platform for DMD
However, the optogenetic model of DMD can also be used to study the pathophysiology of DMD myofibers and identify potential therapeutic targets. In their paper, Yung-Yao Lin and his co-workers used it to elucidate the role of TGFβ signalling.
»Previous studies have shown that TGFβ signalling regulates skeletal muscle homeostasis and that TGFβ signalling is abnormally upregulated in DMD skeletal muscle. Nevertheless, the role of TGFβ signalling in neuromuscular connectivity remains unclear. Our study found that genes regulating neuromuscular junction assembly and axon guidance were dysregulated in DMD myogenic cultures. However, inhibition of TGFβ signalling normalises the expression of many of these genes leading to a beneficial effect for motor function,« explains Amaia Paredes-Redondo.
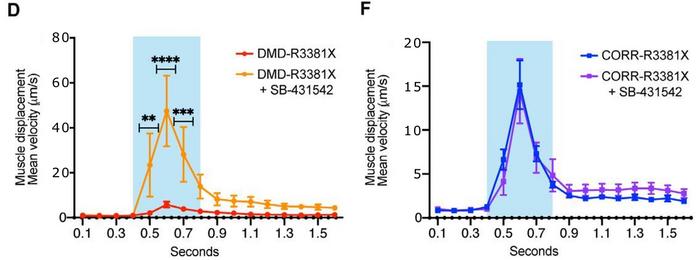
This was shown by applying the selective inhibitor of TGFβ signalling, SB-431542, to myofibers in the optogenetic model. The treatment had no effect in CRISPR-corrected myofibers, while SB-431542 treatment in un-corrected, mutated myofibers increased contraction velocity significantly. Furthermore, the research team used the human neuromuscular circuit co-cultures to develop a 96-well high-content imaging-compatible drug screening platform for DMD cellular phenotypes.
CRISPR plays an integral part in DMD research and therapy
»Our study provides a novel human micro-physiological neuromuscular model for studying neuromuscular defects in DMD and evaluating drug candidates or CRISPR-based therapeutics. Furthermore, our findings suggest that enhancing neuromuscular connectivity may be an effective therapeutic strategy,« concludes Yung-Yao Lin.
“I believe that novel CRISPR technologies hold great promise in developing future medicine”Yung-Yao Lin
He points out that CRISPR can not only be used as a curative tool in DMD but also as a valuable technology for developing other treatments:
»Without CRISPR-based genome editing, it would have been difficult and time-consuming to generate an isogenic control in our study. The current development of CRISPR-based technologies has been very rapid and innovative. I believe that novel CRISPR technologies hold great promise in developing future medicine.«
Link to the original article in Science Advances:
[This article was updated on 24 October 2022 in response to an erratum published by the authors of the original research. A calibration error in particle image velocimetry analysis resulted in y-axis scale 1000 times higher.]
Tags
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.