Catch up on CRISPR COVID-19 Diagnostics
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
When we talk about CRISPR and genome editing, the potential to cure devastasting genetic diseases and cancer quickly come to mind. Indeed, CRISPR therapies for these diseases are advancing fast with a number of CRISPR clinical trials in diverse disease areas progressing through the clinic, and the first cures for sickle cell anaemia and beta-thalassemia are already within reach.
In spite of their vast potential, CRISPR-based diagnostics have received less attention than CRISPR therapies over the years, but this changed with the onset and continuence of the COVID-19 pandemic, which has led to massive innovation and technological advancements that we continue to cover as they emerge.
Last year we covered the first wave of CRISPR diagnostic tools such as ‘The world's first FDA-approved CRISPR application’ by Sherlock Biosciences, A 'CRISPR-Cas13 Surveillance Chip’ for massive testing developed at the Broad Institute, and the 'CRISPR-chip' set to revolutionise biology and help fight the COVID19 pandemic. Since then, we have covered two additional inventions - a ‘lab-on-chip’ for viral detection and a CRISPR face mask. These latest inventions are highlighted in this piece.
CRISPR lab-on-a-chip detects COVID-19 in just over half an hour
In December 2020, we interviewed Ashwin Ramachandran and Juan Santiago, both at Stanford University, whose expertise in microfluidics led to the development of a novel electric field-driven lab-on-a-chip that could detect SARS-CoV-2 RNA in nasal swabs in just 35 minutes.
The setup exploits the same basic principle used in other CRISPR-Cas12a-based viral detection methods. Briefly, Cas12a is guided to a target DNA sequence, in this case reverse-transcribed SARS-CoV-2 RNA, by a gRNA.
Once Cas12a binds the target, it becomes activated, allowing it to non-specifically cleave a fluorescently-labelled reporter DNA probe which is also added to the reaction. Detection of the cleaved probe thus provides a readout for the presence of the target, in this case SARS-CoV-2.
Unlike previous CRISPR-Cas12a approaches to SARS-CoV-2 detection which tend to require multiple separate steps with many moving parts, the new setup uses a selective ionic focusing technique called isotachophoresis (ITP) to perform automated purification of the target RNA from raw nasopharyngeal swabs. An amplification step is performed outside the microfluidic chip, and the amplified material is then applied to the chip in a very small space (approximately 100 picoliters in volume) that also contains a concentrated Cas12a-gRNA complex and fluorescent reporter molecules.
The tiny scale greatly reduces the amount of material needed, reduces cost, increases fluorescent signal intensity and allows the reactions to proceed very fast, with a readout in 35 minutes from start to finish.
The team validated their setup using PCR-tested samples with no false positives and a positive prediction rate of 93%.
At the time of our interview, Ramachandran and Santiago told us that they were working to incorporate the amplicifcation step into the chip as well. The work was published in PNAS with collaborators from other departments at Stanford University.
Read our full interview with Ashwin Ramachandran and Juan Santiago here.

CRISPR face mask can detect COVID-19 and other pathogens
In June, we interviewed Peter Nguyen, research scientist at Harvard University’s Wyss Institute for Biologically Inspired Engineering. Together with James Colllins, also at the Wyss Institute and Harvard, Nguyen led a diverse team from Harvard, Massachusetts Institute of Technology, and DREAMLUX, a fabrics manufacturer in Italy, to develop a shelf-stable biosensor comprised of freeze-dried cell-free synthetic circuits loaded with CRISPR enzymes.
In a remarkable synthetic biology feat that builds upon years of knowledge amassed in Nguyen’s and Collins’ labs, the team found that upon embedding the sensors into garments, armbands and face masks, it was possible to detect pathogens or other molecular targets with high sensitivity.
In the setup, the wearable devices are activated upon rehydration from aqueous exposure events and the presence of specific molecular targets is detected through colorimetric changes in a substrate or via an optical fiber network that detects fluorescent and luminescent outputs.
Compared to the quantitative PCR that is the current gold standard for COVID-19 testing, the team found that face masks embedded with CRISPR Cas12a designed to detect SARS-CoV-2 reverse-transcribed RNA could detect a COVID-19 infection with equal precision.
The mask first gathers aerosolised samples into a contained area, and then a single button rehydrates and reactivates all of the biological machinery, triggering a series of lysis, amplification, and CRISPR detection reactions that all take place on that same sensor pad at room temperature. Turnaround time from mask application to readout is 60-90 minutes, but the researchers are working to shorten this time and reduce cost, as well as working on a mask that can discriminate between different SARS-CoV-2 variants.
The findings could eventually have huge implications for diagnostics as well as environmental safety monitoring and poison detection, as Peter Nguyen told us: »Any metabolites, any toxins, any nerve agents, we can detect that as well. We think this is the first demonstration that opens this huge box of tools that we can use for robust wearables.«
The findings were published in Nature Biotechnology on June 28th 2021.
You can read our full interview with Peter Nguyen, published on the same day, here.
Innovative diagnostics continue to emerge
Just a few days ago, research led by James Collins at the Wyss Institute and Harvard was published in Science Advances describing a low-cost, CRISPR-based detection device that uses saliva samples to diagnose specific COVID-19 variants within just one hour.
The new platform, called minimally instrumented SHERLOCK (miSHERLOCK), is a tabletop device designed for point-of-care diagnostics, which reports the presence of SARS-CoV-2 mutants by sending feedback through a smartphone app. The app uses the phone's camera to detect fluorescence that indicates a positive or negative result, and it is possible to collect test data for large-scale variant surveillance.
The detection method builds upon SHERLOCK, a CRISPR-Cas13a platform that has been used to create highly sensitive diagnostics for a number of viruses including Zika and SARS-CoV-2. miSHERLOCK is the first point-of-care approach to detect SARS-CoV-2 mutants, including the B.1.1.7 (Alpha), B.1.351 (Beta), and P.1 (Gamma) variants.
The miSHERLOCK workflow requires just two milliliters of saliva that is placed into a collector containing lysis reagents, and upon activation of the device's heater, the RNA within the saliva is concentrated and purified within 3 to 6 minutes. The purified sample is then transferred to a reaction chamber - this is where the Cas13a-based SHERLOCK reaction occurs - and the result is ready 55 minutes later.
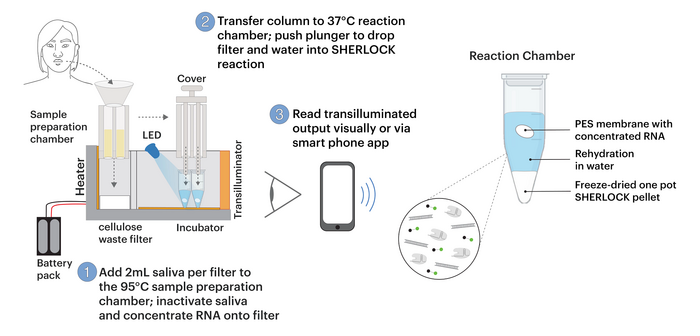
miSHERLOCK is 3D printed, is cheap to produce and is reported to perform equally well to PCR detection. The team behind miSHERLOCK are now working towards extending the platform to detect the so-called Delta variant.
Read more
In case you missed it, you can read our previous roundup on CRISPR COVID-19 diagnostics during the first year of the pandemic here.
If you would like to read about diagnostics application for CRISPR beyond COVID-19 detection, you can find our diagnostics interviews right here.
Tags
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







