CRISPR-Cas9 Knockout of a Novel Cancer Checkpoint Unleashes T Cell Reactivity Against Solid Tumours
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...

Teamwork can unleash big achievements, and, in this case, it has led to a new CRISPR-based cancer therapy that just entered clinical trial.
» I was fortunate to have at my disposal great mentorship and a very talented group of administrators, scientists, GMP-specialists and quality control folks that were all working together for a common goal,« says Douglas Palmer, co-lead-author of the study that was recently published on the bioRxiv preprint server.
Palmer holds a PhD in immunology and biochemistry and has worked for almost two decades at the National Cancer Institute (NCI) in Bethesda, Maryland. He is devoted to innovating and translating novel immunotherapies for the treatment of cancer, and much of the teamwork he refers to has been carried out with cellular and molecular biologist Beau Webber and immunologist/genome engineer Branden Moriarity at the Masonic Cancer Center at the University of Minnesota.
CISH is a Negative Regulator of Immunity
Together, the researchers and their colleagues have used the CRISPR/Cas9 gene-editing technology to inactivate a gene called cytokine-inducible SH2 (CISH) in tumour infiltrating lymphocytes (TILs) and thereby increase their capability to fight solid tumours. At the NCI, Palmer first demonstrated in a paper published in 2015 that CISH is a negative regulator of immunity, and he believes that this prevents exuberant overreaction to, e.g., chronic infections.
»Cancer is similar in some respects to chronic infection, with low and persistent antigen exposure often over many years. When we looked at T cells inside the tumour, we observed that CISH was upregulated,« Palmer explains and elaborates:
»This early work was done in mice since CRISPR technology was still in its infancy, and we were relegated to using knockout mice and siRNA to study the role of CISH in tumour immunity.«
But as the gene-editing technology started to mature, Webber and Moriarity joined forces with Palmer. Moriarity recalls:
»We were collaborating with the NCI on engineering T cells, and once our capabilities in T cells with CRISPR were highly efficient, we came up as a group with this collaborative idea to edit CISH in tumour infiltrating lymphocytes (TILs), rather than just peripheral blood T cells.«
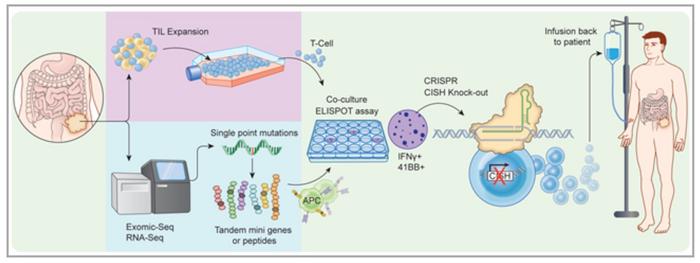
Feeder-Free Activation Step Saves Expensive CRISPR Reagents
The researchers used several algorithms to design gRNAs and evaluated these for changes in function and knockout efficiency by Western blotting.
»We tested about a dozen gRNAs targeting different exons in CISH and identified those that gave the most robust protein knockout. Then we selected a subset that were predicted to have low off-target activity. With a further subset of those, we used the GUIDE-Seq method developed in 2014 and subsequent deep sequencing of predicted off-target sites. At the end, we identified a lead gRNA with no detectable off-target editing « explains Moriarity.
With intensions to take the new approach into the clinic, the team had to optimise the strategy and achieve clinical scale, GMP compliant CRISPR/Cas9 editing of TIL. That was very challenging since the expansion of TILs typically occurs with an excess of feeder cells that you don’t want to electroporate with your expensive GMP CRISPR reagents.
»To solve this problem, we had to modify the standard procedure by incorporating an initial feeder-free activation step prior to electroporation. This allowed us to electroporate a relatively small amount of TILs and then transfer them to feeder co-culture for expansion,« says Webber and adds that with this approach they can achieve over 95% efficiency of CISH knockout in fully mature and expanded human TILs.
Having the protocol in place, the team validated the CRISPR/Cas9 editing process on TILs from a small number of patients with gastrointestinal cancers. In in vitro experiments, they found that CISH knockout significantly increased and extended the proliferation of TILs and that the modification also had the desired effect of enhanced tumour neoantigen reactivity.
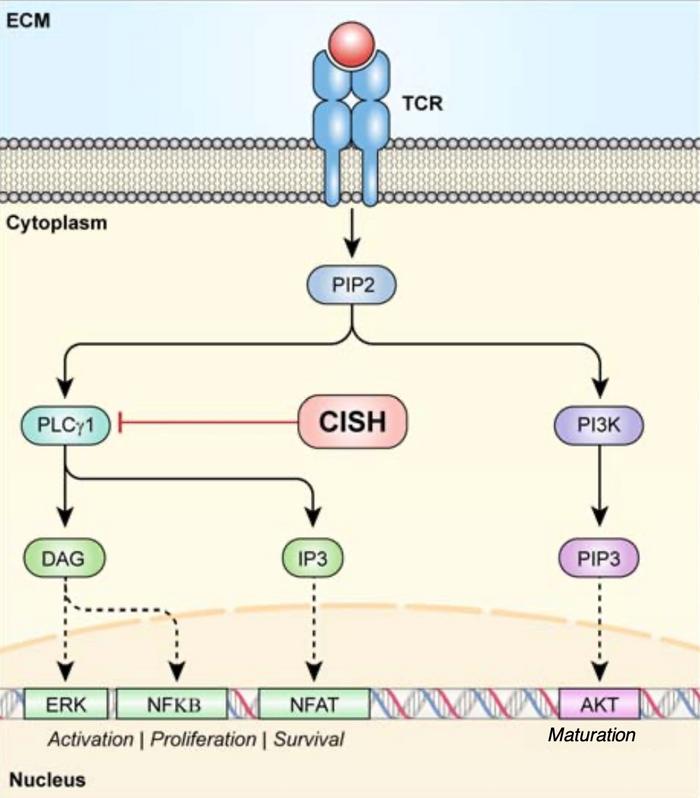
It is Like Putting More Soldiers on the Battlefield
»When a T cell comes in contact with its target, say a neoantigen-expressing tumour, there ensues a signalling cascade resembling a pyramid, with each progressive step enlarging T cell activation,« Palmer explains and continues: »CISH blocks one of these early steps by targeting PLCG1 for degradation, thus blocking the conversion of PIP2 into IP3 and DAG. IP3 and DAG are important secondary messengers in eliciting a potent T cell response and the knockout of CISH facilitates their hyperactivation and subsequent T cell activation.«
CISH is an entirely new target for checkpoint inhibition in immunotherapy. Several clinical studies are under way to evaluate the effect of CRISPR-Cas9 mediated knockout of another immune checkpoint protein, PD-1. For example, a CRISPR trial for advanced lung cancer demonstrated safety of PD-1 knockout. So having the opportunity to also target CISH opens up new avenues for treatment.
Moreover, the teams at the NCI and the University of Minnesota found that CISH-deficient T cells worked synergistically with a PD-1 antibody blockade. This was shown in a preclinical mouse model where the cocktail resulted in durable tumour regression and survival of tumour-bearing animals.
PD-1 is an extracellular checkpoint, and antibodies that target the protein are already approved and widely used in immunotherapy. In contrast to PD-1, however, CISH is an intracellular target that accordingly cannot be targeted by antibodies.
»For me, the most exciting part of this study is that when we knockout CISH, it basically unlocks reactivity to neoantigens that would otherwise not be detected. So instead of delivering a TIL therapy that may have two or maybe three reactive T cells, now we might have 10 or 20 reactive T cells. That is like putting more soldiers on the battlefield,« says Moriarity.
“It has been the biggest research “high” of my career to be able to translate my findings into patients”Douglas Palmer
To Palmer, the most fascinating aspect of the study was that it conceptualises how T cells in your body can migrate to your tumour and reside there in a somewhat dormant state as the tumour grows.
»The ability to reliably gene-edit and significantly enhance the reactivity of these fully mature T cells blows my mind every time we do it. It has been the biggest research “high” of my career to be able to translate my findings into patients. My hope is that this therapy and ones following it can provide more than just hope to those suffering from this terminal disease,« he says.
CISH Knockout has Moved into a Clinical Trial
That hope might not be so far from reality since the work has now progressed into a phase I/II clinical trial in patients with metastatic gastrointestinal cancer at the University of Minnesota. The trial opened in May and is currently enrolling patients. But first, the team had to go through an extensive approval process with regulatory agencies.
»We essentially generated a scaled-up GMP-compliant manufacturing process at the University of Minnesota. And we had to assemble the chemistry, manufacturing, and control (CMC) section of the investigational new drug (IND) application and work with the FDA to answer their questions before we were given approval,« Webber explains.
The clinical trial will assess the safety and efficacy of the CISH knockout approach and reveal if unleashing the functional capability of T cells that are specific for tumour neoantigens could result in off-tumour toxicities. Palmer believes that the strategy’s design will result in a positive outcome:
»TILs from gastrointestinal cancer patients are largely oligoclonal, and we select ones with a predominance of neoantigen specificity. We hope that knocking out CISH in these selected TILs will negate any potential off-tumour effects,« he says.
CRISPR Medicine News also interviewed gastrointestinal oncologist Emil Lou who is leading the clinical trial that has opened exclusively at the University of Minnesota.
Link to the original article on the bioRxiv preprint server:
Internal checkpoint regulates T cell neoantigen reactivity and susceptibility to PD1 blockade
Tags
ArticleInterviewNewsElectroporationGastro-Intestinal Cancer, GICRISPR-CasCas9Clinical
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







