CRISPR Delivery To The Skin With Microneedles
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...

Using microneedles for transdermal delivery of CRISPR-Cas9 and glucocorticoids proved to be a viable way to precisely treat inflammation caused by conditions like psoriasis and atopic dermatitis.
Most gene therapies so far have focused on internal organs like the kidney or liver, but a team of scientists came up with a new tool for administering CRISPR-Cas9 for skin disorders, potentially making local administration much simpler and more precise.
By loading up a new fully-dissolvable patch of microneedles with a CRISPR-Cas9 gene therapy and other therapeutic compounds, the researchers say they've developed a direct way to treat skin-related genetic disorders in vivo — without the needles even reaching the patient's pain receptors. When the microneedles enter the skin, they rapidly dissolve to release Cas9 ribonucleoproteins that get taken in and distributed by nearby immune cells.
That led to a targeted treatment for inflammatory skin disorders (ISDs) in a mouse model. While the microneedle patch hasn't yet been tested in humans, it shows great potential as a new way to administer transdermal gene therapies directly to the affected area. According to preclinical, proof-of-concept research published in the journal Science Advances in March, the new delivery system proved to be a viable way to deliver gene editing.
Inflammatory skin disorders on the rise
Atopic dermatitis (more commonly known as eczema) and psoriasis are chronic inflammatory skin disorders for which there is presently no cure. Without an effective therapy already in place, patients of both disorders are often left managing symptoms like dry, itchy, or cracked skin and trying to prevent or mitigate cyclic flareups through skincare and by carefully managing their diets. Psoriasis patients, in particular, also deal with swollen or painful joints and patches of seemingly scaly skin.
But both inflammatory skin disorders are hereditary and linked to specific alleles and mutations. While scientists still don't fully understand the specific genotypes that lead to psoriasis and atopic dermatitis, that genetic component makes them prime candidates for new gene therapies through CRISPR or base editing.
»We realized that inflammatory skin disorders such as psoriasis and atopic dermatitis are one of the most persistent diseases and are becoming the major issues threatening public health with increasing prevalence. Currently, patients who suffered from these disorders are limited to a few approved therapeutic options. Most of them often show inadequate responses to topical or systemic glucocorticoid therapy, the first-line treatment modality, as a result of glucocorticoid resistance,« says Dr Yuan Ping.
He is a gene-editing drug delivery specialist from the College of Pharmaceutical Sciences at China's Zhejiang University and the last author of the Science Advances paper.
Two disorders with genetic roots in common
Treating the two ISDs together makes a great deal of sense. Researchers have uncovered that psoriasis and eczema's genetic roots are a series of surprisingly similar mutations. A literature review published in the International Journal of Molecular Sciences in 2017 showed that, among the many underlying molecular factors and environmental triggers behind psoriasis, many versions of the disorder share the same gain-of-function mutations in the CARD14 gene that result in the upregulation of inflammatory cytokines. Meanwhile, researchers found that eczema can be caused, in part, by similar mutations in the CARD11 gene that leave patients vulnerable to inflammatory infections.
Inflammasomes
Inflammasomes are innate immune system receptors and sensors that regulate the activation of caspase-1 and induce inflammation in response to infectious microbes and molecules derived from host proteins. They have been implicated in a host of inflammatory disorders. The NLRP3 is expressed predominantly in macrophages and as a component of the inflammasomes that are primarily located at the epidermal and dermal layers of the human skin.
However, Ping and his team focused on another similarity between eczema and psoriasis — an inflammasome called Nod-Like Receptor family, Pyrin domain-containing 3 (NLRP3), implicated in several autoimmune and inflammatory skin disorders. Activating the NLRP3 inflammasome leads to a greater inflammatory response through programmed cell death alongside the overexpression of encoding caspase-1 (CASP-1), increasing a patient's resistance to anti-inflammatory medications.
»Thus, we wish to find an alternative approach to improve glucocorticoid therapy and started to work on microneedle-assisted genome editing of the NLRP3 gene for the treatment of inflammatory skin disorders,« Ping says.
Taking advantage of CRISPR-Cas9 to disrupt NLRP3 the idea is to break the glucocorticoid resistance and improve sensitivity to the glucocorticoid therapy given simultaneously.
Microneedle: A gentle one-two punch
There's no reason that an in vivo gene therapy for ISDs couldn't be injected through a traditional needle. But the dissolvable microneedle patch does come with specific benefits worth exploring to treat skin conditions, Ping explains, especially when it comes to children with skin disorders who might be nervous around or otherwise difficult to treat with frequent and sometimes painful injections. The local transdermal administration also cuts down on the risk that the Cas9 might make off-target edits, improving the overall safety of the gene therapy for the patient.
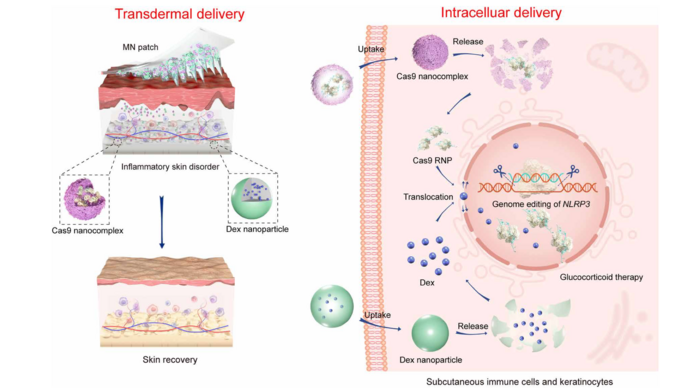
Schematic illustration of stepwise transdermal and intracellular delivery of genome-editing agents (Cas9) and glucocorticoids (Dex) to treat inflammatory skin disorders. From Science Advances
»The microneedle patch can be placed on the skin for the treatment of ISDs. The barely visible microneedles are so tiny that they do not reach the pain receptors, making the treatment relatively painless. In contrast, conventional methods such as local injection of therapeutics are generally painful, which are not suitable for populations, such as children,« Ping says.
Clinicians using the patch would also be able to administer an all-in-one treatment, Ping explains, combining the gene therapy with other therapeutics, like the anti-inflammatory corticosteroid dexamethasone, tailored to the patient's symptoms.
»In addition, the microneedle contains collagen tripeptide and can contribute to the tissue repairment of skin lesions, promote collagen synthesis, and reduce transepidermal water loss. These functions integrated into the microneedle patch may greatly benefit the treatment for patients with ISDs,« he says.
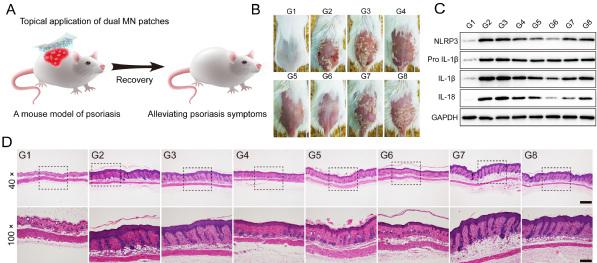
The figures show the treatment effect of various formulations, including normal mice (G1), normal mice with induced psoriasis but without therapy (G2) and normal mice with induced psoriasis and with dual-loaded CRISPR+glucocorticoid microneedle patch (G6). Figures from Science Advances.
That one-two punch of using CRISPR-Cas9 proteins to make a patient's immune system more responsive to the other medications loaded into the same microneedle patch tends to confuse others in the field, Ping explains. But the new transdermal route of administration holds a lot of promise for these previously uncurable skin disorders.
»When I talk about this work, people always asked me whether the treatment of ISDs represents an alternative gene therapy modality. Instead, the microneedle patch contains two types of 'drugs,' including Cas9 ribonucleoprotein and dexamethasone. Whereas Cas9 ribonucleoprotein can target the NLRP3 gene to disrupt the NLRP3 inflammasome, dexamethasone serves as a glucocorticoid agent for the treatment of ISDs. Thus, Cas9-mediated genome editing contributes to improving the sensitivity of glucocorticoid therapy, and the successful treatment of ISDs is a result of combination therapy, instead of a single treatment,« says Ping.
Gene editing into the skin - how far are we?
If you ask Ping about the future of these microneedle patch gene therapies, ISDs like eczema and psoriasis are just the beginning.
»We believe the microneedle patch we have developed would be an accessible route of the administration for gene therapy of other conditions, such as skin cancer and infectious skin diseases. As the microneedle [patch] plays a significant role in the local delivery of therapeutics, it is best suited for skin conditions that transdermal administration is required. Some reports have also shown that [the] microneedle patch can be used to deliver therapeutics to regulate the blood glucose level for the treatment of diabetes,« Ping says.
Meanwhile, Ping and the other researchers have been hard at work exploring some of those other use cases. In the months since they published the ISD paper, they've already developed another patch that Ping says uses CRISPR activation technology to treat infectious skin disorders. Specifically, it seems to be able to reduce bacterial resistance against antibiotics.
But before any of that — either of the existing patches or any that the researchers might develop for other conditions — makes a difference in a single patient's life, a great deal of research and testing still needs to happen. Ping's team hasn't yet pursued clinical research, he explains, saying that there's a long way to go before they might be able to even consider testing the patch on a human.
The mouse models themselves present a close approximation to human psoriasis and atopic dermatitis. The researchers were able to recreate the disease's gene expression patterns found in the human epidermis as well as the patches of thickened skin caused by the mutations' impact on the immune system. But an animal model, no matter how accurate it may be, is still just a model, and there's no guarantee that the patch will work as well in people as it does in mice.
»Before the microneedle patch can be applied to humans, researchers need to ensure the precision and safety aspects of therapeutic genome editing. Although genome editing technologies have made tremendous progress from basic research to preclinical developments in recent years, some challenges, such as off-target effects, still exist. The therapeutic genome editing on human requires more active joint efforts from scientists, clinicians, and bioethicists to ensure that this breakthrough technology can be used safely to treat a variety of diseases,« Ping says.
»The question we would always like to answer is how our research can contribute to a safe and effective gene therapy. To this end, we always think about how genome editing can be safely conducted by rationally designing delivery vectors,« Ping says.
Link to original article in Nature Medicine: Microneedle-assisted genome editing: A transdermal strategy of targeting NLRP3 by CRISPR-Cas9 for synergistic therapy of inflammatory skin disorders.
Dan Robitzski is a science journalist and former neuroscientist based in Los Angeles.
Meet Yuan Ping at the upcoming CMN Webinar - CRISPR Delivery Systems,May 26th 2021
Tags
ArticleInterviewDeliveryIn vivoMicroinjectionAtopic dermatitisPsoriasisBase editorsCRISPR-CasCas9
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







