CRISPR Therapy Turns a Corner With First Cures In Sight
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
At the American Society of Hematology (ASH) meeting last weekend, Vertex Pharmaceuticals and CRISPR Therapeuticspresented new clinical trial data showing consistent and sustained health improvements in a handful of patients treated for two inherited blood disorders with their ex vivo CRISPR-engineered cell therapy, CTX001.
CTX001 is being co-developed by the two US-based companies as a one-time curative treatment for severe sickle cell disease (SCD) and blood transfusion-dependent beta-thalassemia (TDT), two distinct inherited blood disorders that arise from genetic defects in the oxygen-carrier haemoglobin.
The ongoing Phase 1/2 studies are the first CRISPR gene-edited therapy trials to be sponsored by US-based companies, and the latest data raises real hopes for a cure for patients for which current treatment options are sparse, marking a major milestone in the CRISPR medicine field.
Haydar Frangoul, medical director of paediatric hematology and oncology at Sarah Cannon Research Institute in Tennessee, US who treated Victoria Gray, the first SCD patient to receive CTX001 17 months ago was quoted as saying: »We have ameliorated the symptoms. Every time I call her on the phone or see her in the clinic, she feels great.«
Frangoul was also the lead author on a peer-reviewed article written with Vertex, CRISPR Therapeutics and other clinical partners describing Victoria Gray’s treatment as well as one beta-thalassemia patient who received CTX001 in the New England Journal of Medicine (NEJM) a few days ago.
The news has also caused a lot of excitment in the CRISPR research community. Fyodor Urnov, Scientific Director at Innovative Genomics Institute and Professor at UC Berkeley described the sustained clinical benefit of CTX001 as 'a success beyond anyone’s wildest dreams' and said that 'a comprehensive editing-based cure in the next decade is realistic' in a Twitter thread following the publication in NEJM.
Haemoglobinopathies And Foetal Haemoglobin
Haemoglobinopathies are a family of autosomal-recessive disorders that implicate haemoglobin, the red blood cell-borne oxygen carrier. SCD is a group of diseases in this category that exhibits defective adult haemoglobin function, and is associated with a range of life-limiting complications. Beta-thalassemia is also a haemoglobinopathy and belongs to the thalassemias, which are characterised by deficient rather than defective haemoglobin.
Beta-thalassemia is the most common thalassemia and may arise from >200 known mutations in the beta globin (HBB) gene that result in a shortage of mature and functional adult haemoglobin.
The only cure for SCD and the thalassemias is a high-risk bone-marrow transplant, but for most patients the chances of finding a suitable donor are extremely low. Treatment is life-long and includes symptom management, pain relief and blood transfusions.
Foetal haemoglobin (HbF) is highly expressed and critical during foetal development, and is then rapidly suppressed early in life. Reactivation of HbF expression has emerged as an attractive strategy to treat the symptoms of SCD and beta-thalassemia by compensating for the lack of functional adult haemoglobin.
CTX001 Reactivates Foetal Haemoglobin (HbF)
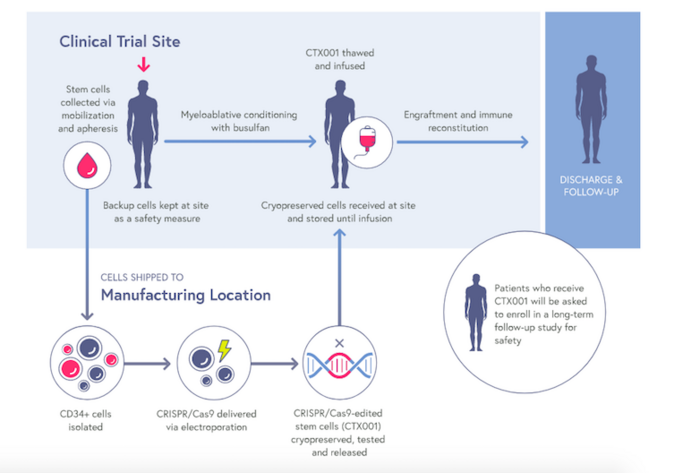
CTX001 is an autologous, ex vivo cell therapy that is made from patient’s own haematopoietic stem cells. The stem cells are first collected from a patient’s blood, and CRISPR-Cas9 is then used to make a small deletion in the B-cell lymphoma/leukaemia 11A (BCL11A) gene within the genome of these cells.
BCL11A is a negative regulator of HbF expression, thus its disruption increases HbF levels, and this is expected to lessen the clinical symptoms of beta-thalassemia and SCD. The patients are given chemotherapy to eradicate their diseased cells, and the BCL11A-knockout stem cells are then infused back in to establish a permanent supply of healthy red blood cells that can produce HbF.
Stuart Orkin, a stem cell biologist at Boston Children’s Hospital, whose lab discovered the BCL11A switch that since paved the way for therapeutic HbF reactivation strategies by Vertex, CRISPR Therapeutics and others described the new results as ‘really very impressive’.
Latest Trial Data Shows That CTX001 Is Efficacous and Safe
CLIMB-Thal-111 and CLIMB-SCD-121 are both open-label Phase 1/2 trials designed to assess the safety and efficacy of a single dose of CTX001 in patients ages 12 to 35 with TDT or severe SCD, respectively.
In June 2020, Vertex and CRISPR Therapeutics released preliminary, mainly positive safety and efficacy data from the first two patients enrolled in these trials, and we covered these updates in a previous article. Since then, a total of 13 TDT patients and 6 SCD patients have been dosed CTX001 and the latest results demonstrate potential efficacy and durability among a larger population of patients with these diseases.
The New Data - Efficacy, Durability and Safety
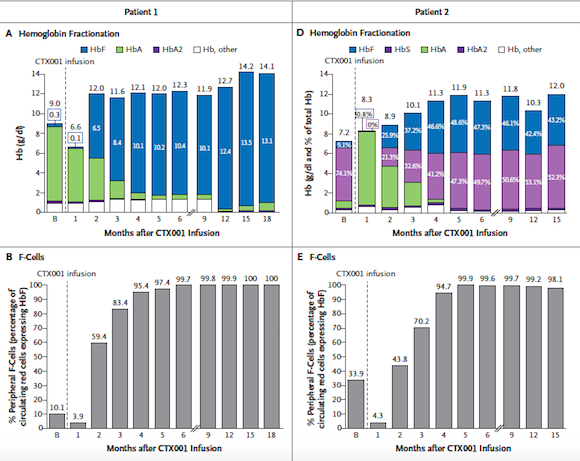
At the ASH meeting, 7 TBT patients were reported to be transfusion-independent with follow-up ranging from 3 to 18 months after a single CTX001 infusion. All 7 patients had normal to near normal total haemoglobin levels at the last checkup, including total haemoglobin from 9.7 to 14.1 g/dL and foetal haemoglobin from 40.9% to 97.7%.
Data was presented for 3 SCD patients who had reached at least 3 months of follow-up after CTX001 adminstration. These patients showed similar response patterns, with rapid and sustained increases in total haemoglobin and foetal haemoglobin, as well as elimination of vaso-occlusive crisis (VOCs), a serious complication of SCD that results when sickled red blood cells obstruct the blood vessels, at the last analysis.
For both trials, bone marrow allelic editing data, which is a measure of the success rate of gene editing, was collected from a selection of patients with 6 or 12 months of follow-up after CTX001 infusion demonstrated a durable effect.
In the peer-reviewed article in NEJM, the authors write: »These findings, which indicate that CRISPR-Cas9–edited HSPCs underwent engraftment that was durably maintained, are consistent with an expected survival advantage of erythrocytes with a high level of fetal hemoglobin«. The authors also point out that: »The clinical course of both patients supports our conclusion that CTX001 mimics the phenotype of hereditary persistence of fetal hemoglobin levels.«
In general, the safety data from all 7 TBT and 3 SCD patients was consistent with an autologous stem cell transplant and the prior chemotherapy to removed diseased cells. Four serious adverse events considered related or possibly related to CTX001 were reported in 1 patient, but which later resolved, and the majority of non-serious adverse events were considered mild to moderate. There were no SAEs considered related to CTX001, and the majority of non-serious adverse events were considered mild to moderate.
A Milestone For CRISPR Medicine Field
The CTX001 trials, which are still ongoing, were originally designed to enroll a total of 45 patients each, but according to an interview covered elsewhere, David Altshuler, Chief Scientific Officer at Vertex Pharmaceuticals is quoted as having said: »I think we could move very quickly here. More patients want to get the therapy and we don’t think we need that many patients for approval. With this kind of effect, we likely need fewer people.«
In a press release about the new data, Reshma Kewalramani, M.D., Chief Executive Officer and President at Vertex said: »These are the first published results from CRISPR/Cas9 therapy in people with a genetic disease and represent an important milestone in medicine and for our collaboration with CRISPR Therapeutics. Most importantly, these data represent a critical step in our effort to bring transformative and potentially curative therapies to patients. With clinical proof-of-concept for both beta thalassemia and sickle cell disease and 19 patients dosed, we look forward to continued efforts to bring our investigational treatment to patients living with TDT and SCD as quickly as we can.«
Tags
ArticleNewsin vivoBeta ThalassemiaSickle Cell Disease, SCDGene therapyCas9SafetyTrialsClinical
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.