First cancer patient dosed in CRISPR-armed phage therapy
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
The Phase 1b trial builds upon encouraging Phase 1a safety data in healthy volunteers where SNIPR001 demonstrated promising safety profiles and successful target engagement.
"This milestone builds upon the highly encouraging data from our CARB-X funded Phase 1a trial in healthy volunteers, where SNIPR001 demonstrated promising safety and target engagement," says Dr Christian Grøndahl, Co-founder and CEO of SNIPR Biome.
The therapy addresses a critical clinical need in haematological cancer patients, who face significant risk of life-threatening infections during treatment. Escherichia coli causes 25-30% of bacteremia cases in neutropenic haematological cancer patients, with up to 65% of bloodstream isolates showing fluoroquinolone resistance.
Current prophylactic treatments with broad-spectrum antibiotics contribute to further resistance development and disrupt beneficial gut microbiota.
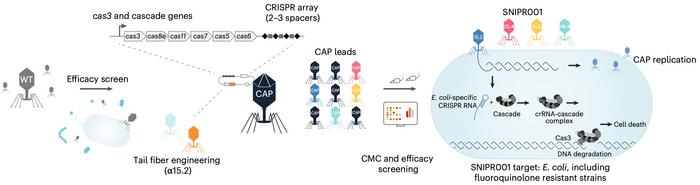
SNIPR001 combines four engineered bacteriophages (CAPs - CRISPR-CAS armed phages) that specifically target fluoroquinolone-resistant E. coli in the gut microbiome (see Figure 1). This precision approach was developed through systematic screening of 162 wild-type phages against 429 phylogenetically diverse E. coli strains, followed by sophisticated engineering that incorporates both tail fiber modifications and CRISPR-CAS machinery to enhance targeting specificity and reduce resistance development. The therapy's precision approach offers potential advantages over broad-spectrum antibiotics by selectively eliminating pathogenic E. coli while preserving beneficial gut microbiota.
"SNIPR001 represents a scientifically innovative strategy that could contribute to addressing an urgent clinical challenge in preventing drug-resistant infections in vulnerable cancer patients," says Dr Erin Duffy, Chief of Research & Development, CARB-X.
The Phase 1/2 trial, co-funded by CARB-X, marks a significant advancement in the clinical development of engineered phage therapies for combating antimicrobial resistance in high-risk patient populations. Read the press release from SNIPR Biome here.
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleBreaking newsCMN BriefsNewsIn vivocrPhageCancerHaematological malignancies (blood cancers)Bacterial InfectionBlood diseaseCas3SNIPR Biome
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







