Gene-Editing IND and Pre-clinical Update
Allogene Therapeutics, San Franscisco, is developing a pipeline of off-the-shelf allogeneic (donor-derived) CAR T-cell therapies for a range of haematological and solid cancers. This update looks at two of the candidates, one of which is at the investigational new drug (IND) stage, i.e. pre-clinical studies are complete and the candidates have been cleared by regulatory authorities for clinical testing in human subjects.
Both of these candidates, ALLO-316 and ALLO-605, are developed using Allogene’s proprietary AlloCAR T platform.
AlloCAR T Platform
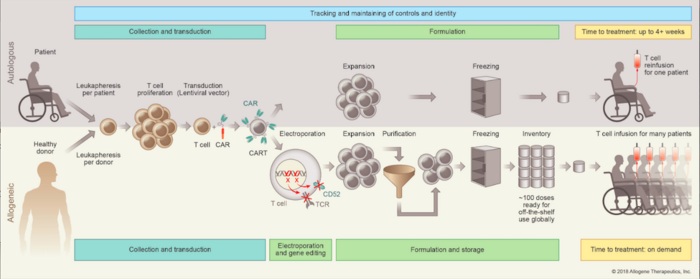
The ALLO CAR T candidates begin with T cells from healthy donors. These cells are engineered using TALEN gene-editing technology to express chimeric antigen receptors (CARs) that can recognise certain cell-surface proteins that are expressed on haematologic or solid tumours, as well as activate T cells to destroy the cancer cells.
Among the major advantages of using donor-derived cells as opposed to using patient-derived cells (as is the case with autologous cell therapies) is that there is a much larger source of cells to work with, making it possible to develop consistent off-the-shelf-therapies, while sparing patients the need to undergo blood cell isolation extraction procedures.
Mitigated Unwanted Immune Responses to the Therapy
The downside of using allogeneic cell therapies is that the risk of graft-vs-host disease (GVHD) must be mitigated to avoid rejection of the therapy and to protect patients. GVHD may result from histo-incompatibility between a donor and recipient and can be fatal. To alleviate this risk, the T cell receptor (TCR) gene, TRAC, in the donor-derived cells is disrupted using TALEN technology.
The CD52 gene is also disrupted using TALEN technology. This is a common feature for allogeneic CAR T therapies, in which an anti-CD52 antibody is administered prior to CAR T therapy to suppress the patient’s immune system and allow the CAR T cells to stay engrafted for full therapeutic effect. CD52 disruption renders the CAR T cells resistant to this treatment.
Finally, when the modifications are complete, the engineered T cells are purified and cryopreserved in vials for on demand delivery to patients.
ALLO-316 – IND for Renal Cell Carcinoma
ALLO-316 is an anti-CD70 AlloCAR T™ candidate that is being developed to treat renal cell carcinoma (RCC) as well as several haematological cancers that express the cell-surface antigen CD70. These cells contain a CD70-targeting CAR that is TALEN-engineered under licence using Cellectis’ TALEN® gene-editing technology. CD52 is also disrupted for the reasons outlined above.
Allogene presented pre-clinical data for ALLO-316 in models of acute myeloid leukaemia (AML) at the recent American Hematology Society (ASH) annual meeting. Specifically, they reported that CD70 expression was detected on AML cell lines and primary samples from AML patients, but not in haematopoietic stem cells. ALLO-316 was able to kill leukaemic cells in multiple animal models, and this activity was abolished in CD70-deficient models. While activated T cells usually express CD70, the company reported that ALLO-316 CAR T cells did not express CD70, likely because the CAR masks CD70 on the cell surface. This is important because it enhances persistence by minimising the risk of the ALLO-316 cells targeting themselves.
Allogene also announced at the ASH meeting that the FDA had cleared an IND application for ALLO-316 for a Phase 1 clinical trial in patients with advanced or metastatic RCC. This trial is expected to start enrolment early in 2021 and will be the company’s first solid tumour clinical trial.
ALLO-605: Pre-Clinical Programme for Multiple Myeloma
ALLO-605 is a TurboCAR™ being developed for multiple myeloma (MM). This candidate is TALEN-engineered to target the B cell maturation antigen (BCMA), a cell-surface protein universally expressed on malignant plasma cells.
ALLO-605 is Allogene’s first TurboCAR™ T cell therapy candidate, which in addition to the gene-editing workflow described above is further engineered to stimulate cytokine production in a ligand-binding independent manner. This feature is designed to increase the potency of the AlloCAR T cells by enhancing their proliferation and overcoming T cell exhaustion.
At the ASH annual meeting earlier this month, the company presented pre-clinical data for ALLO-605, which demonstrated enhanced cytokine secretion, polyfunctionality, improved serial killing activity in vitro, and enhanced anti-tumour activity and survival compared to standard BCMA CAR T cells in an aggressive mouse model for multiple myeloma.
Allogene also revealed at this meeting that it anticipates filing its first IND application for its novel TurboCAR™ technology in the first half of 2021.
For a complete overview of current gene editing clinical trials, check out CRISPR Medicine News' Clinical Trials Database.
Tags
Articlein vivoMultiple Myeloma, MMRenal Cell CarcinomaSolid TumoursCAR-TTALENsTrialsIND - Investigational New DrugPre-clinical
CLINICAL TRIALS
Sponsors:
National Institute of Allergy and Infectious Diseases (NIAID)