Highlight: DNA Breaks Facilitate Transfer of mtDNA into the Nuclear Genome
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
A recent study led by Jiazhi Hu at Peking University reports that DNA breaks in either the nuclear or mitochondrial genomes can facilitate the integration of mitochondrial DNA (mtDNA) fragments into nuclear DNA. The researchers focused on understanding this integration process, especially in the context of CRISPR gene editing, examining how it may influence genome stability and raise considerations for CRISPR-based therapeutic applications.
Using various human cell lines, primary T cells, and murine embryos, researchers observed that both nuclear CRISPR-Cas9 editing and mitochondrial gene editing prompt mtDNA integration into nuclear genomes at target sites. Additional stresses, like drug-induced mitochondrial damage, further increased the frequency of these transfers.
The study, published last week in Nature Communications, examined nuclear-mitochondrial DNA fusion across multiple CRISPR target sites. It revealed mtDNA integration events occurring at rates up to 1% of the edited cells, with slightly lower integration frequencies observed for Cas12 nucleases (see Figure 1).
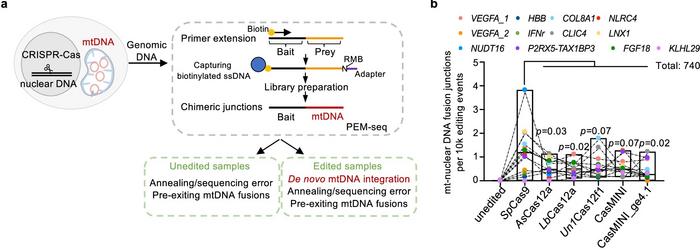
The study found that even high-fidelity CRISPR-Cas9 variants did not stop mtDNA fragments from integrating into nuclear DNA, indicating that the occurrence of DNA breaks—rather than nuclease specificity—is the primary factor enabling these integrations. Breaks in nuclear DNA appear to provide sites where mtDNA fragments can be inserted, regardless of whether they originated from editing or natural processes. This may potentially affect genomic stability and raise concerns about unintended effects in gene therapies.
To counter this issue, the researchers included the exonucleases TREX1 and TREX2 with mitochondrial gene-editing tools. By degrading free mtDNA fragments in the cytoplasm, these enzymes effectively lower the likelihood of their integration into nuclear DNA breaks. This method significantly reduced mtDNA integration events, offering a promising approach for increasing the safety of CRISPR-based treatments (see Figure 2).
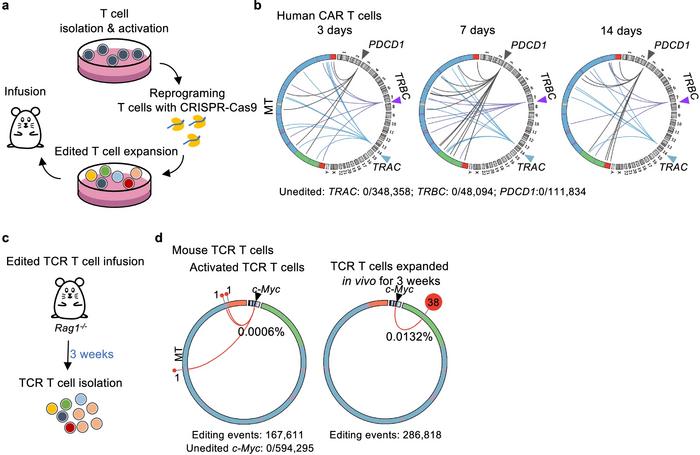
This research highlights critical considerations for gene-editing applications, particularly in therapeutic settings where genomic integrity is paramount. The integration of mtDNA into the nuclear genome could introduce unintended mutations or alter cellular function, presenting risks for patient outcomes. By showing how TREX1 and TREX2 can limit these events, the study suggests an effective strategy for safer gene-editing applications and points to the need for ongoing research into genome stability in CRISPR interventions.
Gene editing of mtDNA was performed with both mitochondrial base editors (such as DdCBE) and mitoTALEN - a mitochondrial-targeted gene-editing technique designed to introduce double-stranded breaks (DSBs) selectively within the mitochondrial genome using transcription activator-like effector nucleases (TALENs) engineered to localise within mitochondria.
Link to the original article in Nature Communications:
Wu et al. (2024) Transfer of mitochondrial DNA into the nuclear genome during induced DNA breaks. Nature Communications, 1 November 2024, doi.org/10.1038/s41467-024-53806-0
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







