Highlight: In Utero Nanoparticle Delivery Enables Brain Gene Editing
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
Researchers from the University of California, Davis and the University of California, Berkeley have used a mouse model to demonstrate a high-efficiency approach to in utero gene editing. The method overcomes traditional barriers by safely transfecting neural stem cells with CRISPR-associated mRNA that can reach large swathes of the brain and introduce precise genetic edits with significant developmental benefits.
The researchers demonstrated that intracerebroventricular (ICV) injection of acid-degradable PEGylated LNPs (ADP-LNPs) leads to high transfection rates and a safety profile compatible with prenatal intervention that might be promising for tackling disorders like Angelman syndrome and Rett syndrome before they manifest (see Figure 1).
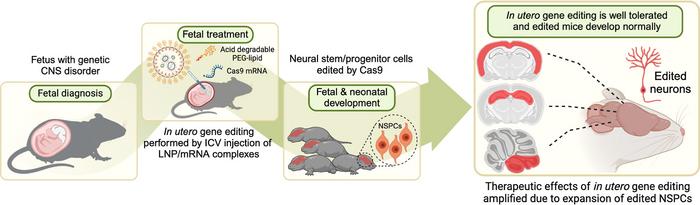
While standard LNPs are effective in adult applications, they have shown limited transfection efficacy and significant toxicity in developing tissues. ADP-LNPs address these limitations with dense PEGylation, which improves cellular uptake while reducing inflammatory responses and acid degradability, which allows these particles to break down within cells after delivering their genetic payload.
In this study, ADP-LNPs containing either Cre mRNA (for the Ai9 genetic reporter system) or Cas9 mRNA paired with guide RNAs (gRNAs) targeting specific genes were injected into the brains of embryonic mice on day E15.5, a period when neural progenitor cells are actively proliferating.
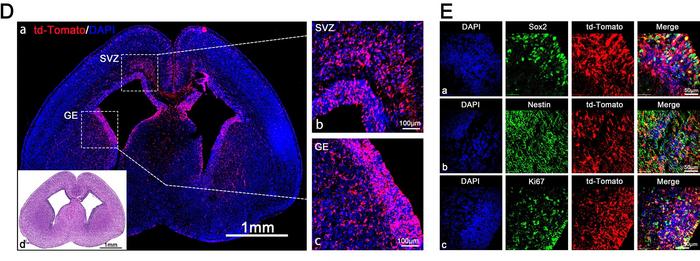
The results were impressive: the ADP-LNPs achieved widespread gene editing across the brain, with approximately 30% of fetal brain cells transfected. Edited neural progenitor cells continued to proliferate throughout development, ultimately modifying over 40% of neurons in the cortex and more than 60% of neurons in the hippocampus by 10 weeks after birth (see Figure 2).
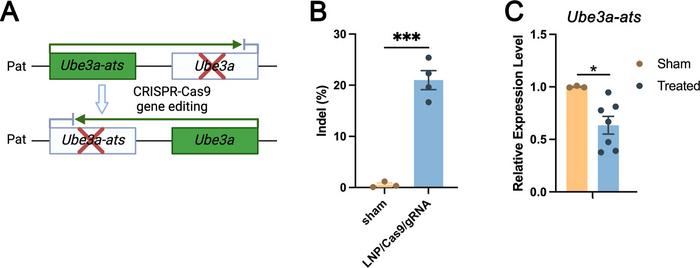
To potentially treat Angelman syndrome, the researchers constructed ADP-LNPs carrying Cas9 mRNA and a gRNA specific for knocking out the disease-causing Ube3a-ats locus. This locus produces an antisense transcript that silences the expression of Ube3a, a ubiquitin ligase essential for brain development (see Figure 3).
Injected into fetal mice, the ADP-LNPs were able to achieve 21% gene editing in targeted brain cells within one week postpartum and a significant reduction in Ube3a-ats expression. This is a much higher level of precision and efficiency than previous delivery systems have been able to achieve and a significant improvement in both the scope of tissue transfection and the maintenance of cell viability and function.
The study’s rigorous approach included multiple assays to confirm safety and specificity. Unlike standard LNPs, which prompted high inflammation and lowered survival rates, ADP-LNPs kept cytokine levels nearly at baseline in the brain and liver. Mice treated with ADP-LNPs also showed normal growth patterns and healthy weights by 10 weeks of age.
The findings suggest that ADP-LNPs could potentially enable in utero preventive therapies that might revolutionize treatment options for many currently untreatable neurodevelopmental disorders.
Link to the original article in ACS Nano :
Gao et al. (2024) Widespread Gene Editing in the Brain via In Utero Delivery of mRNA Using Acid-Degradable Lipid Nanoparticles. ACS Nano, 24 October 2024, doi.org/10.1021/acsnano.4c05169
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleCMN HighlightsNewsDeliveryDelivery - Brainin vivoLipid-based nanoparticleAdeno-associated virus (AAV)Angelman SyndromeCas9
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







