Highlight: Tripartite complex boosts non-viral gene knock-in accuracy
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
Gene therapy holds enormous promise for treating various diseases by inserting therapeutic genes into patients' cells. However, efficiently and safely integrating large DNA sequences into precise genomic locations has remained challenging, particularly when trying to avoid viral vectors with their associated safety concerns.
Researchers at Full Circles Therapeutics and collaborating institutions addressed this challenge by creating what they call "enhanced GATALYST-associated genome editors" (enGagers). These are engineered Cas9 proteins fused with single-stranded DNA binding domains - either full-length proteins like RecA or compact 20-amino-acid peptide motifs derived from these proteins (see Figure 1). The team demonstrated that these fusion proteins significantly improve the efficiency of integrating circular single-stranded DNA (cssDNA) templates into targeted genomic locations.
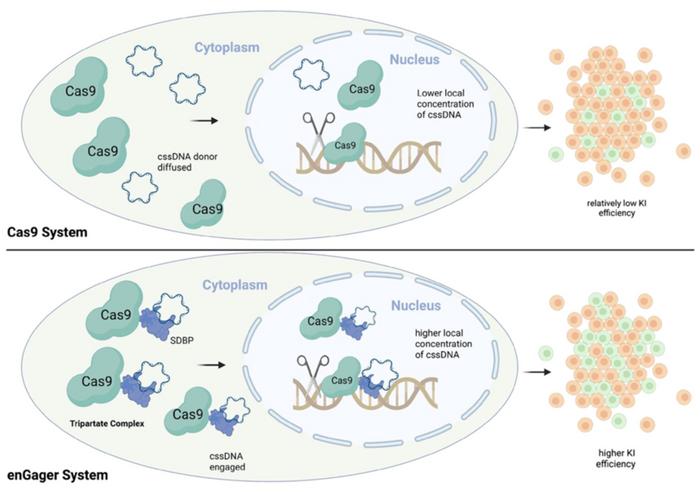
Crucially, the researchers found that adding even just a 20-amino-acid single-stranded DNA binding motif to Cas9 was sufficient to enhance knock-in efficiency comparably to fusion with full-length proteins. This is significant because smaller modifications to Cas9 are less likely to interfere with its core editing functions. They named this streamlined system 'TESOGENASE' (tripartite editor with ssDNA optimized genome engineering).
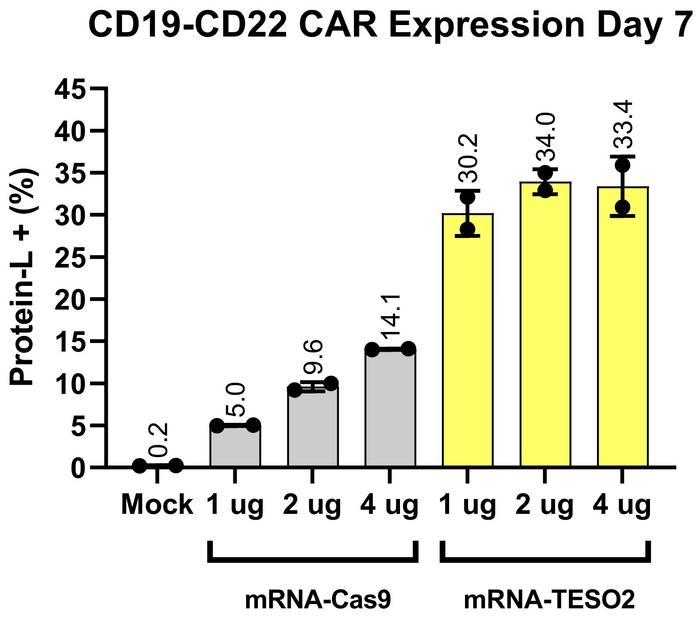
The enhancement effect was demonstrated across multiple cell types and genomic loci, working impressively with large DNA cargoes that have traditionally been difficult to integrate. The system showed particular promise in primary human cells, where it achieved efficient integration of a chimeric antigen receptor (CAR) transgene in 33% of primary T cells - a critical application for cancer immunotherapy (see Figure 2).
Importantly, the enGager system only enhanced integration when using single-stranded DNA templates, not double-stranded DNA, confirming the mechanism relies on the specific binding of single-stranded DNA. Additional experiments demonstrated that the system increases nuclear localization of DNA templates, thereby increasing the local concentration of donor DNA at the integration site.
The technology represents a promising non-viral alternative for therapeutic gene modification, addressing many of the safety issues associated with viral vectors in gene therapy applications.
Hao Wu from Full Circles Therapeutics and Jiahe Li from the University of Michigan led the research, and it was published in Nature Communications on May 16.
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleCMN BriefsCMN HighlightsNewsElectroporationCAR-TssDNACas9Full Circles Therapeutics
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







