IND Update: First Clinical-Stage CRISPR Therapy for HIV
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
Excision BioTherapeutics, a private company that develops gene-editing anti-viral therapies announced last week that the FDA had cleared its HIV candidate EBT-101 for clinical trial initiation in the US. This is the first time that the FDA has given IND approval to a CRISPR-based therapy for HIV.
The company is developing EBT-101 as a potential cure for chronic HIV infection, and the trial will specifically assess the safety, tolerability and efficacy of the new drug candidate in individuals living with HIV type 1, which is the subtype present in about 95 % of HIV cases worldwide.
EBT-101 is developed as an in vivo CRISPR therapy
HIV is an RNA virus, and when it first infects an individual, its RNA is reverse-transcribed into DNA by virally-encoded reverse transcriptase and RNase H enzymes that convert the viral RNA to double-stranded DNA (dsDNA) and degrade any remaining viral RNA, respectively. The resulting dsDNA is integrated into the host genome and this is referred to as proviral RNA.
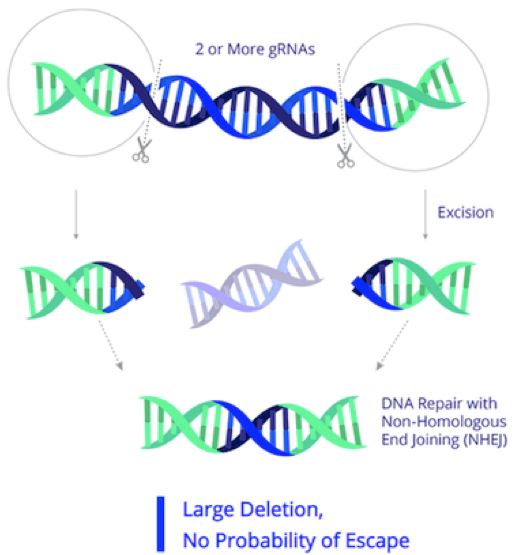
EBT-101 is designed to excise HIV proviral DNA using CRISPR-Cas9 and two guide RNAs (gRNAs). The CRISPR cargo is delivered to cells as a one-time treatment via an adeno-associated virus (AAV). The two gRNAs target three sites within the HIV genome (Figure 1), thereby cutting out large portions of the HIV genome and minimising potential viral escape.
Stay up to date with clinical updates from the CRISPR Medicine field!
For a complete overview of CRISPR IND approvals and ongoing gene-editing clinical trials, check out CRISPR Medicine News' Clinical Trials Database.
Pre-clinical studies demonstrate curative potential
Previously reported pre-clinical studies carried out in the lab of Kamel Khalili at Temple University in Philadelphia, which is associated with Excision BioTherapeutics, demonstrated that EBT-101 could remove HIV proviral DNA from multiple cell lines as well as the genomes of transgenic mice. This was the first demonstration of a functional cure for HIV in animals, and the work was carried out in collaboration with Dr. Howard Gendelman's group at the University of Nebraska Medical Center.
Subsequent proof-of-concept studies shed similar findings in rhesus macaque monkeys infected with the related simian immunodeficiency virus (SIV), where a single systemic treatment with AAV9-delivered Cas9 and dual gRNAs could remove proviral DNA from the genomes of blood cells and other tissues known to be viral reservoirs.
EBT-101 is Excision’s lead programme, and the company’s proprietary viral excision technology was developed at Kamel Khalili's lab at Temple University and Jennifer Doudna’s lab at UC Berkeley.
EBT-101 clinical trial (EBT-101-001)
This is a First in Human (FIH), open-label, sequential cohort, single ascending dose (SAD) study of EBT-101, which will be administered intavenously (IV) to aviremic HIV-1 infected adults on stable antiretroviral therapy (ART).
On Day 1, eligible participants will receive a single IV dose of EBT-101. All participants will be assessed for eligibility for an analytical treatment interruption (ATI) of their background ART at Week 12. All participants will be followed through Week 48, and participants who undergo ATI will have more frequent study visits than non-ATI participants.
Eligible participants who are enrolled in this study will also be enrolled in a separate long-term follow-up (LTFU) study (EBT-101-002) for safety monitoring. The duration of the LTFU study will be up to 15 years.
This article was originally published on 22th September 2021. It was updated on 8th December 2021 to include information about the EBT-101-001 clinical trial.
Tags
ArticleNewsIn vivoAdeno-associated virus (AAV)Human Immunodeficiency Virus Infection, HIVViral diseaseBlood diseaseCRISPR-CasCas9FDAExcision BioTherapeuticsTrialsIND - Investigational New Drug
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







