Israeli researchers are developing a CRISPR gene editing therapy for SCID, interview with Ayal Hendel
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...

Recently Ayal Hendel received a letter with an image of a newborn boy suffering from a rare and life-threatening genetic disorder called SCID or severe combined immune deficiency.
»His uncle asked whether I could use CRISPR to cure the baby,« says Ayal Hendel, at the Bar-Ilan University in Israel where he is developing a CRISPR gene editing therapy for SCID.
Children with the disorder are born without a functional immune system, meaning that any stray microbe picked up from touching another human being or just breathing air can be lethal. The disorder is also called 'bubble boy disease' named after David Vetter who was born in Houston nearly five decades ago, and lived his entire life - 12 years - in plastic chambers or 'bubbles'. (See video at the end.)
»The baby was actually born with the same disease as David Vetter,« says Ayal Hendel.
Luckily doctors have since learned how to perform bone marrow transplants, and with Hendel's colleague, Professor Raz Somech at the Edmond and Lily Safra Children’s Hospital, the baby underwent a successful bone marrow transplant.
“We need CRISPR for these cases with poor babies, who don't have donors,”Ayal Hendel
Unfortunately, a compatible donor is not always available.
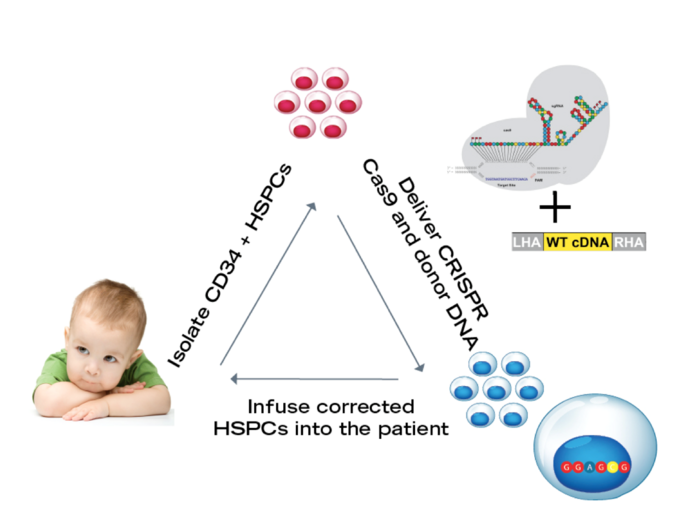
To cure SCID Hendel and his colleagues aim to use CRISPR-Cas gene editing to correct the genetic defect in patient blood stem cells that may be isolated from the umbilical cord blood. The researchers then infuse the corrected stem cells back into the patient where they can develop into a fully functional immune system.
For the past years, Ayal Hendel has been working on developing and optimising the formula for genome editing the blood stem cells or haematopoietic stem cells. He recently published a study together with Integrated DNA Technologies (IDT) on how to perform the editing efficiently and how to assess safety aspects taking the technology a big step closer to clinical trials.
»Right now, we are running more the safety assays to demonstrate that there is no genotoxicity on the DNA level or any other physiological toxicity, « says Ayal Hendel.
»We want to develop the technology as fast as we can to clinical trials».
The work began in Matt Porteus lab at Stanford
Ayal Hendel completed his PhD at the Weizmann Institute in Israel with Professor Zvi Livneh in the field of DNA repair. Then it became clear that he wanted to go to Stanford University with scientists like Robert Lehmann, Arthur Kornberg and Paul Berg who made DNA discoveries foundational to genome editing such as the DNA polymerase, DNA ligase and recombinant DNA.
»For me, Stanford was the Mekka of genome editing,« says Ayal Hendel.
He joined the lab of Matthew Porteus who was the first to show how to use zinc finger nucleases and donor DNA to induce genome editing by homologous recombination in mammalian cells and had just arrived at Stanford from University of Texas Southwestern Medical Center in Dallas, Texas.
Hendel began working with zinc finger nucleases and TALENs, but then CRISPR exploded on to the scene made life a lot easier.
»CRISPR streamlined the development process of making new engineered nucleases, and it democratised the field of genome editing, « says Hendel.
Two papers opened blood stem cells to CRISPR editing
The focus was to engineer haematopoietic stem cells, and together with a young postdoc from Denmark, Rasmus Bak, and a postdoc from the US, Danny Dever, Hendel was involved in two ground-breaking papers that made that possible.
The editing efficiency of haematopoietic stem cells had until then been low, but in the first paper, they showed how to edit the genome efficiently. The trick turned out to be chemical modifications to the guide RNAs, which protects them from getting chopped up by exonucleases within the cells.
In the other paper, they showed how to deliver the donor DNA needed for homologous recombination by adeno-associated virus (AAV) in the blood stem cells.
»Based on these two papers, you had the formulation to use genome editing by homologous recombination in haematopoietic stem cells,« says Ayal Hendel.
There is a wide range of immunodeficiencies and blood disorders that CRISPR gene editing can be applied to, and now with his lab in Israel, Ayal Hendel is focusing on the immune system and particularly SCID.
SCID is a good candidate for genome editing
There are several reasons for this. The first is that SCID is a life-threatening disease and gene editing can be a cure. Technically the gene-editing reagents can be delivered ex vivo - outside the patient's body - giving a lot of control in terms of safety. Furthermore, haematopoietic stem cells have been in the clinic for several years with established protocols for isolation, transplantation and transfusion. One other significant advantage with SCID is that it is only necessary to correct a small number of cells.
»Between 5 to 10% of the haematopoietic stem cells is going to be enough, because they will make more of themselves and then generate enough immune cells in the blood system,« says Ayal Hendel.
Last but not least in terms of reasons to work with SCID is that the incidence of SCID in Israel is more than twice as high as in the US because of higher rates of consanguineous marriages - marriages within the family.
»In the US the incidence of SCID is around 1 in 58,000 versus 1 in 23,000 in Israel,« says Ayal Hendel.
Correcting the RAG genes
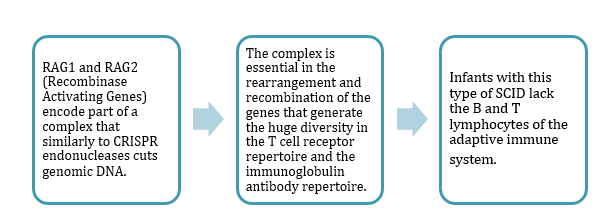
SCID is more than one disease. There are at least 13 different types of genetic defects that can cause SCID, and Hendel's research is focused on correcting one type related to the RAG1 and RAG2 genes (see box).
It is not the most common type, but there is a good reason for choosing the RAG genes.
»One thing makes the RAG genes very unique - the entire coding region of the gene is located in only one exon,« says Ayal Hendel.
»It's important for us because we can design one guide RNA, one CRISPR that will cut the DNA at the very beginning of this exon and replace it with a new wild-type exon.«
In short, this means that there can be one solution which fits all the different RAG mutations.
»No matter which patient is coming, this strategy should work,« says Hendel.
The Hendel Lab continue to optimise the formulation developed at Stanford with the synthetically modified guide RNA.
They harvest stem cells from umbilical cord blood, which is a rich source of haematopoietic stem cells, and deliver the Cas9 as a ribonucleoprotein (RNP) complex with the synthetic guide RNA by electroporation in the lab. Then the donor DNA that harbours the wild-type RAG exon is delivered by AAV.
New faster, more efficient and safer formulation
The team recently published an important new study on the CRISPR-Cas reagents for the RAG2 and RAG1 genes and how to maximise editing efficiency and minimise off-target editing. In other words, how to find the most efficient and safe gene editing needed for therapy.
»For clinical applications, it's crucial to understand what the off-target sites are, « says Ayal Hendel. »We were able to reduce off-target activity to below 0.1 %, and these off-target sites won't prevent us from starting clinical trials. «
According to Hendel, some off-target effects can be acceptable, and he points out that gene therapy for a different type of SCID associated with the ADA gene is based on viruses that can integrate and generate off-target sites.
»Off-target sites are something that we need to study and confirm that they are not in coding genes or generate toxicity. But they won't prevent us from moving to the clinic, « says Ayal Hendel.
He looks forward to developing the technology through preclinical trials to begin the first clinical trials either in Israel or together with centres in Europe or the US within the next few years.
And hopefully, the babies and their families can eventually have a real cure.
VIDEO: Watch the Surprising Legacy of the Boy in the Bubble | Retro Report
Tags
ArticleInterviewAdeno-associated virus (AAV)Severe Combined Immunodeficiency, SCIDGene therapyOff-targetCRISPR-CasCas9
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







