Multiplex CRISPR Could be Universal Path for Resistance to Major Plant Disease
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
CRISPR AgroBio News (CARBON) is a new initiative from CRISPR Medicine News. CARBON will bring you the latest news on how CRISPR can shape agriculture for the future to guarantee food security in times of population growth and climate change.
To get more CRISPR AgroBio News delivered to your inbox, sign up to the free weekly CARBON Newsletter here.

Powdery mildew (PM) is a major fungal pathogen in grains, vegetables and fruits, and global losses amount to several million tonnes per year. In theory, it is simple to breed resistant crops by selecting for loss-of-function mutations in the PM susceptibility gene MLO, but this comes with a substantial growth penalty. Now, Chinese researchers use multiplex CRISPR to simultaneously knock out MLO and activate a dormant gene that fully restores growth and yield in wheat. The strategy is currently being adapted for other crops, and it can pave the way for a general method to make virtually any crop resistant to PM while maintaining yield.
»We tested our CRISPR-edited elite wheat varieties in the field at two locations and in the greenhouse. They are fully resistant to powdery mildew without any growth penalties,« says Caixia Gao, a professor and principal investigator at the Institute of Genetics and Developmental Biology of the Chinese Academy of Sciences in Beijing, China. She is the co-senior author of a paper published online today in Nature, which describes how stacking genetic changes can be used to develop high-yielding, disease-resistant crops.
Gene editing activates gene through chromatin changes
The strategy is based on multiplex CRISPR with two gRNAs. The first gRNA ensures the knockout of all MLO loci, i.e., MLO-A, MLO-B and MLO-C, that each reside on one chromosome set in the hexaploid wheat. MLO is a susceptibility (S) gene required for a successful PM infection. Loss-of-function mutations of all copies of MLO result in durable and broad-spectrum resistance to PM. But regrettably, such mutations also result in cell death, early senescence, and reduced growth.
The second gRNA activates a gene called TMT3 that encodes the tonoplast monosaccharide transporter (TMT), a membrane protein that transfers glucose from the cytosol into the vacuolar compartment. TMT3 is naturally silenced in all tissues except the spike (inflorescence) in wheat. When CRISPR broadly activates TMT3, however, the growth defects caused by MLO knockout are reversed.
“We tested our CRISPR-edited elite wheat varieties in the field at two locations and in the greenhouse. They are fully resistant to powdery mildew without any growth penalty”Caixia Gao
The mechanism behind this is not fully understood, and indeed, Caixia Gao only discovered the interplay between the two genes by accident. In 2014, she used the TALEN gene-editing technology to knock out MLO in wheat and obtained PM-resistant mutants with growth defects. Although she stumbled upon one of these resistant mutants, Tamlo-R32, that grew normally early in the project, it took nearly eight years to elucidate the genetic mechanism behind this mutant’s phenotype. Today’s Nature paper presents an extensive characterisation of this mutant.
To make a long story short, Tamlo-R32 turns out to have a large 304 kb deletion upstream of the MLO-B locus, and this alters local chromatin states. As a result, epigenetic repression is abolished, and a permissive chromatin environment is created around TMT3, which is located just upstream of the deletion on chromosome set B. In other words, the deletion results in an altered local chromatin landscape, leading to the ectopic activation of TMT3, thereby alleviating growth penalties associated with MLO knockout.
Programmed CRISPR gene editing in elite wheat
Today, Caixia Gao demonstrates how the Tamlo-R32 alleles can be recreated with CRISPR in elite winter wheat (Figure 1). CRISPR gene editing was performed with one pair of gRNAs and Cas9 delivered either as DNA constructs or ribonucleoproteins (RNPs) by particle bombardment into around 6,000 immature embryos of four elite varieties. One gRNA targets a conserved site in all three MLO genes and causes their disruption. Another gRNA targets a site 304 kb upstream of MLO-B and causes a large deletion in the B genome that leads to ectopic activation of TMT3.
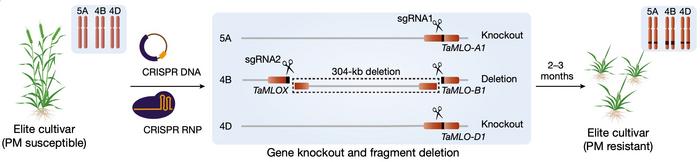
The 304 kb deletion was obtained in 31 mutants across all varieties and delivery methods with frequencies ranging from 0.2% to 0.9%. Among these mutants, 16 were also disrupted in at least one allele in each of the MLO genes, while six were disrupted in both alleles. The latter genotype is required for complete PM resistance since the MLO S genes are recessive. Five of the six mutants with the desired genotype were made by delivery with DNA constructs. Two of them were shown not to have integrated DNA from the CRISPR vector in their genomes, and they belonged to the elite wheat variety Xinong 511.
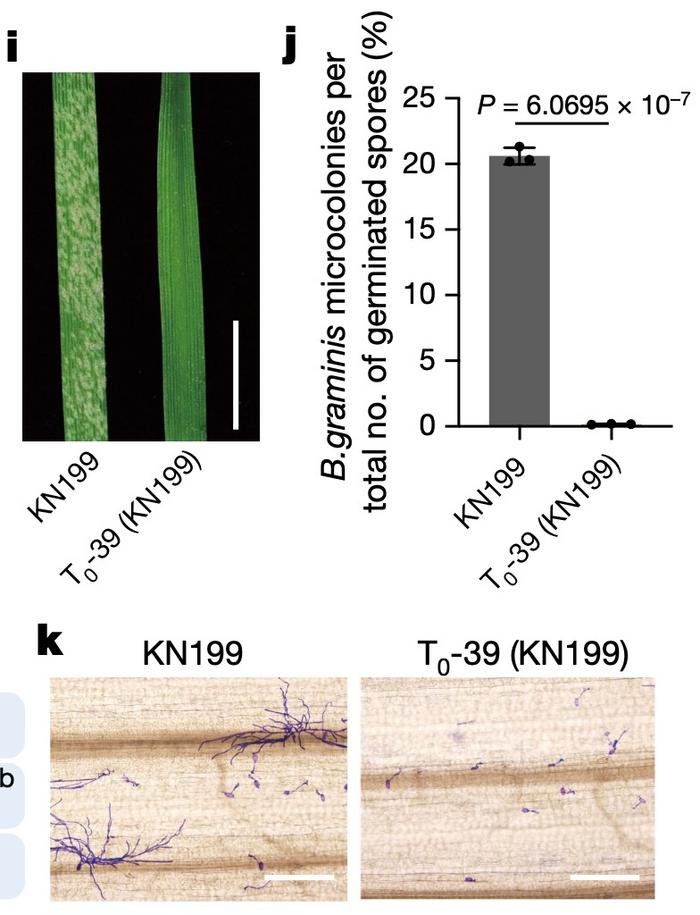
One of the desired mutants, T0-39, was made using RNPs and thus could not possibly contain integrated vector DNA. This was a mutant of the Kenong 199 winter wheat elite variety. When leaves of T0-39 were inoculated with PM spores, virtually no microcolonies formed from germinating spores. On the contrary, microcolony formation was abundant in unedited Kenong 199 (Figure 2).
More crops line up to become disease-resistant
So, delivering Cas9 and the two gRNAs by RNPs should be a straightforward way to develop high-yielding and PM-resistant wheat. The same approach should apply to other crops. In the Nature paper, the researchers show that TMT3 function is conserved in the weed Arabidopsis thaliana and that overexpression of the gene can rescue growth defects in MLO mutants. This is assumed to be the case in other plant species as well.
While it might be straightforward to disrupt MLO in other crops, it will be more challenging to activate TMT3. That is because the gene was activated in wheat due to very specific genomic locations of MLO relative to TMT3, which influenced the local chromatin state and thereby the expression of TMT3. It is highly unlikely that the same conditions will apply in other crops, and therefore deletion of a piece of DNA upstream of MLO is not a realistic approach for TMT3 activation in crops other than wheat.
“In an ongoing follow-up study, we will identify new gene-editing approaches using CRISPR-Cas9 to upregulate TMT3 expression more broadly through both transcriptional and translational activation”Kevin Zhao
»In other crops such as rice, we can’t use the same strategy, but still, we can activate TMT3 with gene-editing technology,« says Caixia Gao. A new postdoc, Kevin Zhao, recently joined her group to follow this lead. He came from David Liu's lab at Broad Institute and was not involved in today's work. But he is now taking the strategy further in other crops.
»In an ongoing follow-up study, we will identify new gene-editing approaches using CRISPR-Cas9 to upregulate TMT3 expression more broadly through both transcriptional and translational activation,« Kevin Zhao explains.
China opens for the cultivation of CRISPR crops
Caixia Gao and Kevin Zhao are now focusing on real-life implementation of the technology and hope to soon see their gene-edited crops growing in the fields. That dream might not be too far away from reality. Just about two weeks ago, on 25 January, the Chinese Ministry of Agriculture and Rural Affairs published new guidelines for the approval of gene-edited plants.
“In 3-4 years, our powdery mildew resistant elite wheat could be grown commercially in the fields”Caixia Gao
According to the new rules, gene-edited plants that have completed pilot trials can apply for a production certificate without the need for lengthy field trials. This effectively paves the way for a fast roll-out of new, gene-edited crops.
»The new policy is very popular, and the whole scientific community was pleased with it. That’s the beauty of this technology. If you can deliver your CRISPR reagents directly to an elite variety, then you are almost ready to move on to the field,« says Caixia Gao. And Kevin Zhao agrees:
»We have plans to put the two gene-edited elite varieties mentioned in the Nature paper forward for regulatory approval under the new policy announced.«
»In 3-4 years, our powdery mildew resistant elite wheat could be grown commercially in the field,« concludes Caixia Gao optimistically.
Link to the original article in Nature:
Genome-edited powdery mildew resistance in wheat without growth penalties
Tags
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







