Nasal Spray Sends CRISPR to the Brain
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...

For over one decade, the Brazilian biochemist Guilherme Baldo has struggled to find new, effective treatments for MPS. This is a group of chronic and progressive genetic diseases that affect several organs, including the brain, which are caused by mutations in genes encoding lysosomal enzymes. Much of his early work was concentrated on enzyme replacement therapies, but just a few years after CRISPR entered the scene in 2012, Guilherme Baldo started to look in that direction.
He is an associate professor at the Federal University of Rio Grande do Sul in Porto Alegre and has extensive experience with the disease subtype MPS I, which is caused by mutations in the ɑ-L-iduronidase (IDUA) gene. In 2018, he and his team were able to demonstrate the feasibility of in vivo CRISPR gene editing in mice using intravenous injection. The treatment partially restored IDUA enzyme activity in organs such as the heart, lung, liver, and kidney, but not in the brain because the blood-brain barrier protects it. And this is a problem for patients with the severe form of the disease who may develop cognitive decline.
»Our main question was, how can we address the central nervous system without invasive approaches like direct injection into the brain? So, we came up with this idea of going through the nose to reach the olfactory bulb right above it,« Guilherme Baldo recalls.
He and his team have now pursued the approach in pre-clinical experiments with mice, and they published their results in The Journal of Gene Medicine earlier this year. The experiments ultimately showed that non-invasive delivery of CRISPR reagents by nasal administration allows for gene editing and partial restoration of IDUA activity in the brain. This, in turn, enhanced cognitive function, while biomarkers of disease in other organs also improved.
Mucopolysaccharidosis I (MPS I)
Mucopolysaccharidosis I (MPS I) is a rare genetic disorder that affects many body systems and that leads to organ damage. It is caused by an alteration in the gene that makes an enzyme called alpha-L-iduronidase. Because of this alteration, cells either produce the enzyme in low amounts or cannot produce it at all. The enzyme is needed to break down substances called “glycosaminoglycans” (GAGs) which are by-products of chemical reactions in the body’s cells. If GAGs are not broken down, they build up in the cell, eventually leading to cell, tissue, and organ damage. From https://www.mps1disease.com/patients/about/what-is-mps1
CRISPR knocks in a functional gene
The team was focusing on a potential clinical therapy from the very beginning of the current study, and that raised two main concerns. First, several different mutations in IDUA can cause MPS I, so to benefit the full spectrum of patients, gene editing must preferably target all these mutations simultaneously. Second, gene editing must be performed in ways that minimise the risks of severe immunological reactions.
“Even though our gene-editing strategy far from normalises the IDUA enzyme levels, we can still expect good clinical effects from the small increases we can reach”Guilherme Baldo
To address the first concern, Guilherme Baldo decided to follow a strategy for CRISPR-directed knock-in of a functional copy of the IDUA gene into a known safe harbour called the ROSA26 locus. This would ensure the expression of the wild-type IDUA enzyme irrespective of the underlying mutation. The researchers addressed the second concern by using non-viral delivery of liposomes containing plasmids encoding Cas9, gRNA and IDUA. Without the need for immunogenic viruses, the liposomes ensure cellular uptake of the gene-editing reagents so they can make a double-stranded break in the safe harbour, and allow the gene to be inserted by homologous recombination.
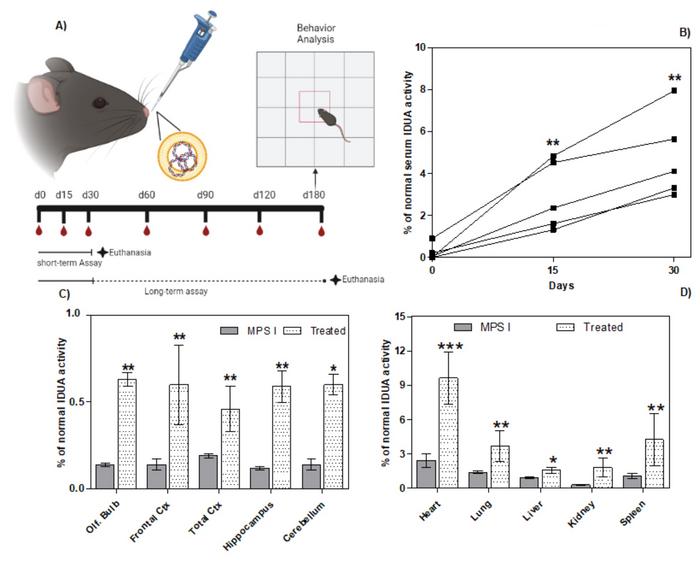
Gene editing experiments were performed in a murine model of MPS I, where the animals are genetically engineered to have the neomycin resistance gene inserted into the IDUA gene, thereby disrupting it. One-month-old mice received daily nasal administration of the liposomal complex for 30 days into each nostril with a sterile pipette (Figure 1). In addition, blood samples were taken regularly to analyse IDUA activity and glycosaminoglycan (GAG) levels. Since IDUA is responsible for the breakdown of GAGs, an increase in IDUA activity and a decrease in GAG levels indicate a therapeutic effect.
A significant increase in serum IDUA activity was seen on days 15 and 30. In a short-term pilot experiment, animals were euthanised at the end of treatment and enzyme activity was measured in different organs and brain areas. IDUA activity was significantly increased in treated versus non-treated animals in all cases. However, the increase came from a very low baseline and reached a maximum of 10% of normal activity. Less than 1% of normal IDUA activity was obtained in the brain.
Gene editing improves cognitive function
Despite the modest increase in enzyme activity, gene-edited mice exhibited improved cognitive performance in a subsequent long-term experiment. Here, the animals were followed for five more months after the 30-day treatment period. At the end of the period, Guilherme Baldo and his colleagues analysed the animals' behaviour in open field tests by observing locomotor activity (crossings) and their willingness to explore the environment by standing on their hind legs (rearings). Patients are typically hypoactive, resembling reduced locomotor activity in mice. These analyses were repeated three times to assess the animals' non-aversive memory, and the results were compared between untreated and treated MPS I mice and normal, wild-type mice.
CMN Webinar: Deliver CRISPR to the Brain - A New Treatment Strategy for Mucopolysaccharidoses
On-demand available here.
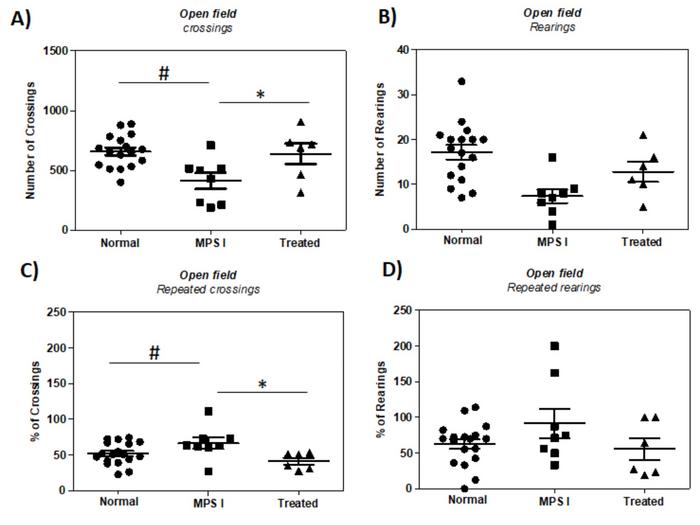
Behavioural results showed that treated MPS I mice had close to normal locomotor activity (crossings), while untreated MPS I mice exhibited a 37% reduction in locomotor activity (Figure 2). In addition, treatment increased the MPS I animals' curiosity and response to stimuli (rearings) from 44% to 75% of normal measurement for these parameters, respectively. In the repeated open field tests, both normal and treated animals showed a higher reduction in crossings than untreated MPS I animals. This indicated that the latter had habituated to the environment to a lesser extent, suggesting a slight improvement in non-aversive memory after gene editing.
»Even with relatively low increases in IDUA enzyme levels, we can get an essential improvement in brain function. This is important for patients with mutations that almost abolish all enzyme activity. As a result, they have a severe form of the disorder and develop cognitive decline and behavioural problems around the first years of age. But we know that patients with less severe mutations - for example, point mutations that still allow for 1-2% residual IDUA activity - tend not to develop brain disorders. So even though our gene-editing strategy far from normalises the IDUA enzyme levels, we can still expect good clinical effects from the small increases we can reach,« Guilherme Baldo says.
Non-human primates are next in line
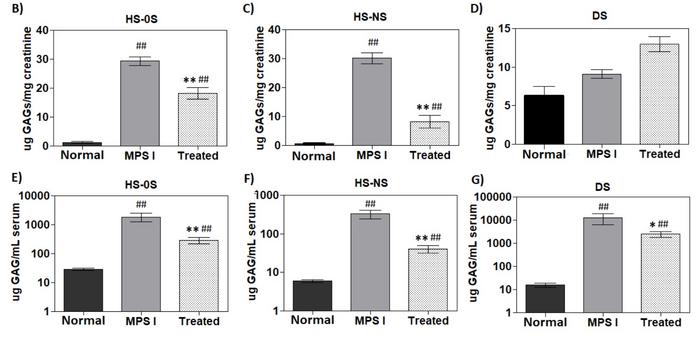
After six months, serum levels of the GAGs dermatan and heparan sulphate, that accumulate in MPS I patients significantly, decreased in gene-edited MPS I mice. However, the levels did not normalise (Figure 3). The decrease in GAGs indicates higher IDUA activity and a positive effect of gene editing. A similar observation was made for heparan sulphate but not dermatan sulphate in urine samples when GAGs were normalised to creatinine. In tissue samples from euthanised animals, gene-edited MPS I mice generally had significantly lower levels of GAGs in different tissues than untreated MPS I mice. This included the brain cortex.
The published study did not analyse the occurrence of potential off-target effects, but the Brazilian scientists are presently sequencing the most likely off-target sites. Guilherme Baldo points out, however, that his team has not observed any increase in tumour frequency in the gene-edited mice.
“I wonder if we can increase IDUA activity to a certain point where we could stop treatment or if we should continue forever. This is something we are now going to study in non-human primates”Guilherme Baldo
The study finds that nasal delivery of liposomal complexes for CRISPR gene editing of the IDUA gene is effective in both the brain and other organs. On the contrary, most other delivery strategies cannot cross the blood-brain barrier and are restricted to gene editing in either the brain or other organs. However, an apparent drawback of nasal delivery is the low efficiency that requires repeated delivery for several days to achieve sufficient gene editing outcomes in mice. But Guilherme Baldo doesn't know for how long treatment will be needed to obtain clinically meaningful results in humans.
»I wonder if we can increase IDUA activity to a certain point where we could stop treatment or if we should continue forever. This is something we are now going to study in non-human primates. We will use another known safe harbour for these studies, namely the CCR5 HIV receptor gene. It has very high sequence similarity in primates, so we can use the exact same vectors and gRNAs in later clinical studies with humans,« says Guilherme Baldo. Mutations or deletions in CCR5 do not lead to any known consequences other than resistance to HIV infection, which could be considered an attractive adverse effect of the gene-editing therapy.
Since the non-human primates are healthy, this upcoming study will primarily address biodistribution, toxicity, and the extent to which repeated administration is required. Guilherme Baldo and his team are now finalising the last details to initiate the gene-editing studies in non-human primates, and he believes they can start in July or August. If all turns out well, the Brazilian team should be able to have the results by the end of this year or the beginning of next year.
Link to the original article in The Journal of Gene Medicine:
To get more of the CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleInterviewNewsDeliveryDelivery - BrainIn vivoNon-viralLipid-based nanoparticleMucopolysaccharidosis I, MPS ICas9
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







