TALEN-Engineered CAR-T Cells Deliver Precision Attack on Solid Tumours
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...

Chimeric antigen receptor (CAR)-T cell therapy has been a revolutionary approach in treating haematological cancers but has shown limited success against solid tumours due to several challenges. Solid tumours present barriers such as cancer-associated fibroblasts (CAFs) in the tumour microenvironment, which prevent T cell infiltration and lead to immune suppression. Moreover, targeting tumour-associated antigens (TAAs) in solid tumours raises concerns given their expression in healthy tissues, risking "on-target, off-tumour" toxicity.
»The problem with solid tumours and CAR-T-cell therapy is that you need a surface protein exclusively expressed on the tumour and not on normal tissues because you want to avoid any off-target toxicity,« says Shipra Das, who is Associate Director at the biotech company Cellectis in New York. She is one of the scientists leading the company's Immune Oncology discovery research group and is the senior author of a new study published last month in Molecular Therapy.
“The idea was to programme a circuit with gates”Shipra Das
To overcome the challenges, her team has developed TALEN-edited allogeneic CAR-T cells that express an IF/THEN logic-gated system. It is a dual-control mechanism designed to activate CAR-T cells only in specific tumour conditions, enhancing precision and reducing off-target effects. Shipra Das explains:
»The idea was to programme a circuit with gates. The first tumour-specific signal would serve as the input, processed by the gate, which would then signal the expression of a CAR that can target a more promiscuously-expressed tumour antigen, which might also be found on normal tissues.«
TALEN was chosen over CRISPR
The system works in two steps. First, a constitutively-expressed CAR targets FAP+ CAFs in the tumour microenvironment (the IF condition). This engagement triggers the expression of a second CAR which targets mesothelin (a TAA) on tumour cells (the THEN response).
The dual-sensing approach ensures CAR-T cell activation only in the presence of both CAFs and tumour cells, improving specificity and minimising damage to healthy tissues (see Figure 1).
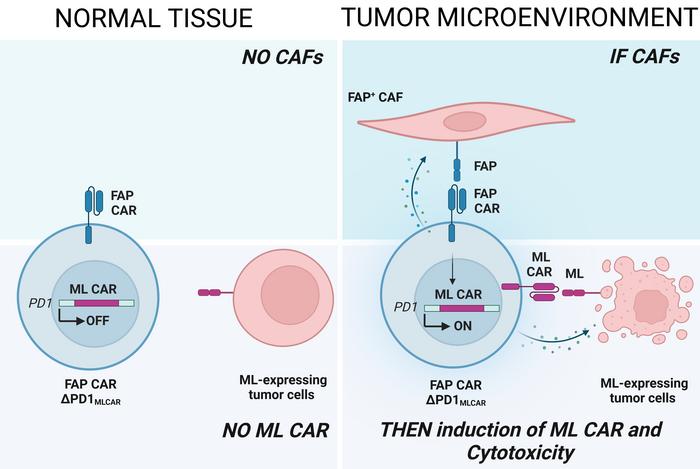
»The idea is to programme multiple modalities into one cell. Targeting CAFs alone hasn't worked well, nor has targeting mesothelin alone. But integrating these modalities into one CAR-T cell creates a more effective immunotherapy,« explains Laurent Poirot. He is Senior Vice President of Immunology at Cellectis in Paris and co-author of the paper.
Besides inserting the CARs, the TRAC and PDCD1 genes were knocked out in T cells, thereby rendering them non-alloreactive and suitable for off-the-shelf use. Knocking out TRAC (T cell receptor alpha constant) prevents the T cells from expressing their endogenous T cell receptor (TCR), thus avoiding graft-versus-host disease (GVHD).
“TALEN is particularly advantageous for specificity because it binds to a longer recognition sequence - 32 nucleotides versus CRISPR's 20-24”Laurent Poirot
PDCD1 encodes the immune checkpoint protein PD-1, which tumours often exploit to suppress T cell activity. By knocking out PDCD1, the engineered CAR-T cells become resistant to this immune suppression, enhancing their anti-tumour activity.
TALEN-mediated gene editing was chosen over CRISPR for knocking out the two genes, as Laurent Poirot explains:
»Both CRISPR and TALEN are highly efficient. But for clinical-grade nucleases, what matters is activity, specificity, and precision. TALEN is particularly advantageous for specificity because it binds to a longer recognition sequence - 32 nucleotides versus CRISPR's 20-24. The longer recognition sequence means fewer off-target effects, which is crucial for avoiding genomic toxicity.«
One CAR activates the other
The CARs were subsequently inserted using different techniques. The FAP CAR (targeting CAFs) was introduced through lentiviral transduction, which stably integrates the CAR gene into the T cells.
The mesothelin CAR (targeting tumour cells) was integrated at the PDCD1 locus using AAV-mediated homologous recombination. This precise integration allowed the mesothelin CAR to be controlled by the same regulatory elements that would normally regulate PD-1, ensuring inducible expression based on the IF/THEN logic-gated system.
“Targeting FAP-expressing CAFs, which are key players in T cell exclusion from tumours, allows the CAR-T cells to further infiltrate the tumour”Shipra Das
Specifically, when the FAP CAR is activated, it initiates a TCR signalling cascade. Since the PDCD1 locus, where the mesothelin CAR is integrated, is regulated by this pathway, FAP CAR activation will lead to surface expression of the mesothelin CAR. So, the FAP CAR serves as a gatekeeper to trigger expression of the mesothelin CAR in solid tumours, but it also serves another purpose.
»Targeting FAP-expressing CAFs, which are key players in T cell exclusion from tumours, allows the CAR-T cells to further infiltrate the tumour. CAFs also modulate the immunosuppressive microenvironment, so disrupting that also disrupts immune suppression in the tumour,« says Shipra Das.
When these dual CAR-T cells engage with their respective targets in the tumour environment, they initiate an immune response by releasing cytotoxic molecules (such as perforin and granzymes) that kill the CAFs and mesothelin-expressing tumour cells.
Survival is significantly increased
The dual CAR-T cells were enriched using magnetic-activated cell sorting (MACS) and extensively tested through various assays, including cytotoxicity, gene expression, and T cell infiltration in 3D tumour spheroids. Moreover, in vivo experiments in mice demonstrated that the TALEN-edited dual CAR-T cells effectively target solid tumours while maintaining safety.
Two mouse models were used. A bilateral tumour model employed mesothelin-positive (ML+) cells implanted in the left flank and a mix of FAP+ CAFs and ML+ cells in the right flank. Another orthotopic triple-negative breast cancer (TNBC) model used TNBC cells and patient-derived CAFs implanted in the mammary fat pad.
In the bilateral model, dual CAR-T cells accumulated specifically in the right flank (FAP+ and ML+), where they activated the mesothelin CAR, showing the IF/THEN logic-gated system's effectiveness. Tumour growth and weight were significantly reduced in the right flank, while no tumour reduction was seen in the left (ML+ but FAP−), demonstrating strong target specificity and a lack of off-tumour effects.
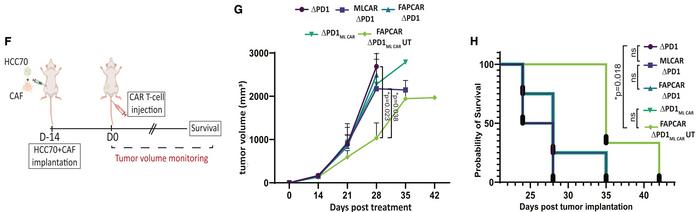
In the TNBC model, the dual CAR-T cells reduced tumour growth and significantly increased survival, proving robust anti-tumour activity (see Figure 2). Crucially, no off-target toxicity was observed in normal tissues, with the mesothelin CAR tightly regulated to be active only in the tumour microenvironment, preventing off-tumour cytotoxicity.
»Our results showed significant tumour reduction where both antigens were present, with no effect on the normal tissue. This was exactly the outcome we hoped for, and we're very happy with the results,« concludes Shipra Das.
Clinical trials might be two to five years away
“Everything here was built with the goal of moving to the clinic, but it's a step-by-step process. Regulatory authorities require a very rigorous demonstration of safety and efficacy before clinical trials”Laurent Poirot
The mice in the study did not achieve complete cures, and according to Shipra Das, this was expected due to the limitations of the model systems used. While the researchers aimed to closely mimic human tumours by incorporating human CAFs and tumour cells, the injected human CAR-T cells might not have the appropriate supportive milieu in the murine environment.
Although the results are encouraging as a proof of concept, future efforts will focus on testing in more physiologically relevant models and optimising the strategy to better tackle the complexity of solid tumours.
»Everything here was built with the goal of moving to the clinic, but it's a step-by-step process. Regulatory authorities require a very rigorous demonstration of safety and efficacy before clinical trials. We'll need to validate everything preclinically before moving into a clinical programme, which could take anywhere from two to five years,« says Laurent Poirot.
Link to the original article in Molecular Therapy:
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleInterviewNewsin vivoCancerSolid TumoursCAR-TTALENsCellectis S.A.
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







