Functionalised Nanocapsules Enable Effective CRISPR Therapy in the Brain
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
The brain is a very delicate organ, and therefore, it is well protected not only from physical damage by the scull but also from harmful chemical solutes and pathogens by the blood-brain barrier (BBB). The BBB is a highly selective semi-permeable border of endothelial cells, and though it effectively reduces the risk of diseases in the brain, it may also reduce the chance of curing them. This is because many medical drugs cannot cross the barrier from the bloodstream, which also applies to most viral vectors, nanoparticles, or other vehicles carrying therapeutic CRISPR reagents.
One way to circumvent the BBB is to inject CRISPR delivery vehicles directly into the brain, but this is a very invasive method that often leads to serious side effects such as infection, inflammation, swelling and tissue injury. Accordingly, the BBB must somehow be penetrated, and this is exactly what Bingyang Shi has achieved in a study published recently in Science Advances. He is an Associate Professor and leads an advanced brain drug delivery group at Macquarie University in Sydney, Australia.
“These nanocapsules are not like a bag you put something into. It's like a rope you wrap around a single Cas9 protein as a customised envelope for delivery”Bingyang Shi
He essentially gift-wrapped single CRISPR-Cas9/sgRNA complexes in a functional coating that served four purposes: protection in the bloodstream, penetration of the BBB, targeting to tumours, and efficient release of the CRISPR reagents inside the cancer cells.
»These nanocapsules are not like a bag you put something into. It's like a rope you wrap around a single Cas9 protein as a customised envelope for delivery. With this approach, we can reach almost 100% loading efficiency, meaning that we can package every single Cas9 protein, each in its own nanocapsule,« Bingyang Shi says. He explains that the approach also accommodates larger complexes and thus can be used for base editing, prime editing, and multiplex gene editing.
Functional molecules keep the nanocapsules on the right track
The coating consisted of a thin polymeric shell made of positively-charged acrylate guanidine crosslinked with N,N-bis(acryloyl)cystamine that contains biodegradable disulfide bonds (Figure 1). The shell was finally decorated with angiopep-2 peptide. While coating rather than encapsulation was chosen to keep the size of the particles down to approximately 30 nm and thereby facilitate BBB penetration, the angiopep-2 peptide served two purposes. It is a ligand that binds low-density lipoprotein receptor–related protein-1 (LRP-1), which is more highly expressed on BBB endothelial cells and glioblastoma (GBM) cells. This will cause the nanocapsules to concentrate around the BBB, increasing their chance of penetration into the brain, where they will subsequently be preferentially taken up by GBM cancer cells.
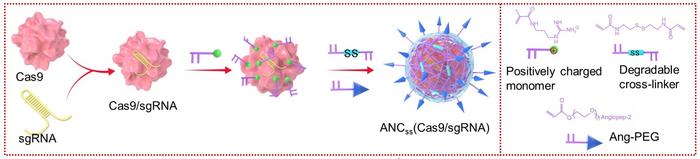
On the other hand, the disulfide crosslinker is sensitive to the higher intracellular glutathione (GSH) conditions present in tumour cells. Here, the high intracellular reductive environment will cause the nanocapsules to degrade by disulfide cleavage, leading to release of the therapeutic cargo.
To test that the functional molecules fulfilled their purposes, Bingyang Shi and his co-workers fabricated three different versions of the nanocapsules. ANCSS contained both the targeting angiopeptide-2 (A) and the releasing disulfide bonds (SS), while ANC only contained A for targeting (in this case, a non-biodegradable crosslinker was used instead of SS), and NCSS only contained the SS moiety for releasing the cargo in cancer cells.
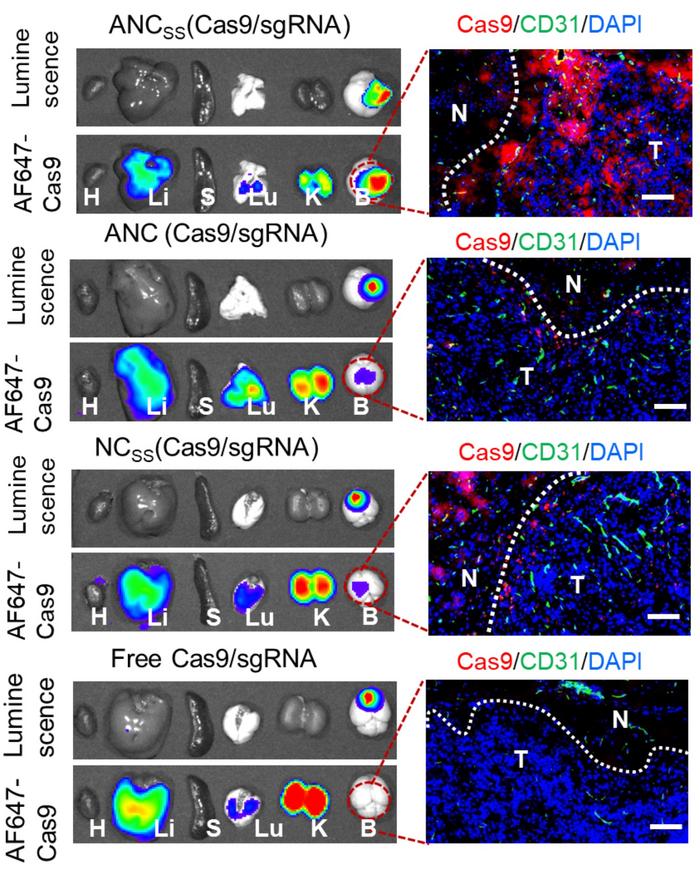
Several in vitro and in vivo experiments – including labelling Cas9 with a fluorescent marker – confirmed that the two targeting nanocapsules, ANCSS and ANC, effectively crossed the BBB and were taken up in tumour tissue significantly better than the non-targeting NCSS (Figure 2). Furthermore, after systemic injection, both ANCSS and ANC accumulated specifically to tumour tissue at levels comparable to the liver and kidney and much higher than in, e.g., the heart and normal brain tissue. The results also indicated that ANC without the biodegradable crosslinker could not effectively release its cargo inside tumour cells.
»In this work, Shi and colleagues rationally designed the system with angiopep-2 peptides for crossing the blood-brain barrier and bioresponsive bonds for releasing the Cas9/sgRNA complexes inside cells. These functional components are critical for the effective delivery of the therapeutic cargo to glioblastoma cells,« says Yizhou Dong at the Ohio State University. He is an expert in drug delivery and did not participate in the study.
GBM tumours are kept in control
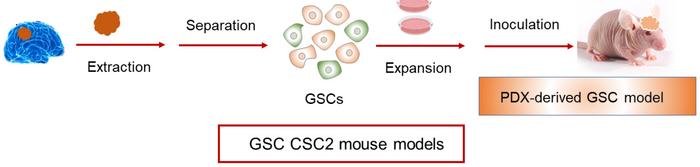
The team then moved to in vivo studies in a patient-derived xenograft mouse model (Figure 3). Briefly, the model was established by separating and expanding GBM stem cells (GSCs) from a patient. Before seeding them in the brain of mice, the GSCs were genetically engineered to express luciferase, so the therapeutic effect of the CRISPR-Cas9 nanocapsules could be easily evaluated. For these experiments, the nanocapsules were loaded with Cas9 and an sgRNA targeting the PLK1 gene. PLK1 plays a crucial role in cell mitosis and is highly overexpressed in GBM cells. Inhibition of PLK1 has been shown to suppress GBM cell proliferation and lead to apoptosis.
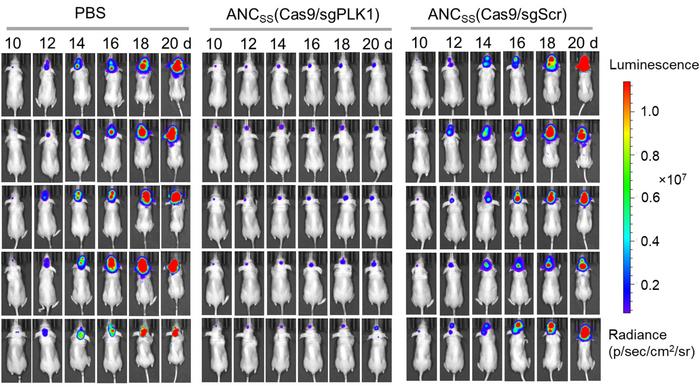
Ten days after implantation of luciferase-labelled GBM tumours, mice were systemically injected with ANCSS nanocapsules carrying either PLK1-targeting sgRNA or scrambled sgRNA as a control. The median survival time was only 24 days for the control animals, while it was significantly extended (p<0,0001) to 68 days for experimental animals with PLK1-sgRNA. Moreover, luminescence images indicated very little tumour growth in therapeutically-treated animals, whereas growth was substantial in mock-treated mice (Figure 4).
The team subsequently verified that the observed therapeutic effects were due to the CRISPR-mediated knockout of PLK1. Firstly, a significant reduction of PLK1 protein expression (around 50%) in treated animals was confirmed. Secondly, Sanger sequencing and the T7E1 assay were used to verify PLK1 disruption. These experiments indicated an indel frequency of 24% and a mutation rate of 28.6% in PLK1. Half of the editing events resulted in out-of-frame mutations.
Bingyang Shi and his colleagues also tested for off-target effects at five potential sites in GBM tumours, and in all cases, mutation frequencies were below 0.5%. The same low mutation levels were also observed for the potential off-target sites in normal brain tissue, liver and kidney. In addition, a series of blood biochemistry and blood parameter analyses showed no difference between animals that had been injected with either just PBS buffer or ANCSS nanocapsules carrying the PLK1-targeting sgRNA. These results suggest a satisfactory safety profile for the proposed therapy for GBM.
Clinical perspectives look bright for the nanocapsules
However, more work is needed before the CRISPR-loaded functional nanocapsules can emerge as a therapy for GBM patients. In the mouse model, a single injection was not enough, and the mice were injected every second day a total of five times. Moreover, the mice eventually died when the therapeutic nanocapsules were no longer injected. This might suggest that humans will need continued treatment, which will not be ideal. To achieve better therapeutic outcomes, Bingyang Shi is already experimenting with multiplex gene-editing of two or three genes that, like PLK1, are also involved in GBM. But the nanocapsules have even bigger potential.
»In this work, we use the glioblastoma cells as an example, but we can apply the technology to other brain diseases as well. We have a couple of projects ongoing to use it for the delivery of CRISPR based treatments for Alzheimer's disease, Parkinson's disease, and motor neuron disease,« Bingyang Shi says.
Yizhou Dong agrees with the versatility of the functional nanocapsules, and he believes that they might also be broadly applicable to various therapeutic agents such as siRNA.
Link to the original article in Science Advances:
To get more of the CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleInterviewNewsDeliveryDelivery - BrainIn vivoLipid-based nanoparticleRibonucleoprotein (RNP)DiseaseCancerCas9
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







