Getting Closer to Single-Treatment Cures at Poseida Therapeutics
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...

As a high school student in a rural school in Hartford, Wisconsin, Eric Ostertag envisioned gene therapy long before it even existed. Later, as a molecular biology student at the University of Wisconsin, and then as the first ever gene therapy graduate from University of Pennsylvania, he dreamed about gene therapies that could cure diseases with a single treatment.
With a PhD and an MD, Eric Ostertag is now heading up Poseida Therapeutics, a 240-strong clinical-stage biopharmaceutical company headquartered in San Diego.
Today, his dream is getting closer to reality with the company’s s expanding CAR T pipeline for a wide range of cancers and gene therapies for congenital diseases.
Two promising autologous CAR T candidates in the clinic
Right now, Poseida has two CAR T candidates in clinical trials for multiple myeloma (MM) and prostate cancer, both of which are derived from patients’ own cells i.e., autologous.
P-BCMA-101 is a Phase 2 candidate for MM that targets the B cell maturation antigen (BCMA). P-PSMA-101 targets the prostate-specific membrane antigen (PSMA) and is at Phase 1 for prostate cancer, and clinical results are exciting so far:
»We’ve already dosed over 100 patients cumulatively in the trials for P-BCMA-101 and P-PSMA-101, and the data is very promising. We see strong response rates, duration of responses and a differentiated tolerability profile compared to other CAR T-cell approaches. Our prostate cancer candidate has shown the best responses ever seen for a CAR T in a solid tumour indication with one patient showing complete tumour elimination and a durable response at 5 months after a single administration of low dose CAR T,« tells Eric Ostertag.
Unlike other companies in the gene-edited CAR T space that are predominantly using CRISPR-Cas for gene editing, Poseida’s unique approach lies in the combination of its proprietary piggyBac® DNA Modification System and the hybrid Cas-CLOVER™ Site-Specific Gene Editing System that offers increased precision over conventional CRISPR-Cas (Figure 1).
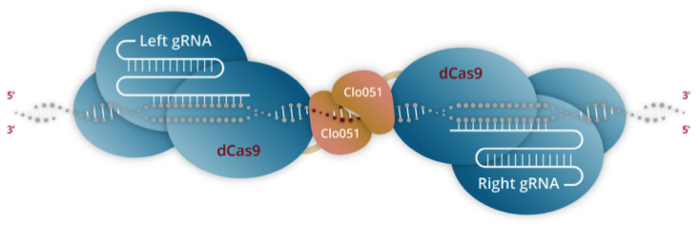
Cas-CLOVER
Cas-CLOVER combines the use of an intact but enzymatically inactivated Cas9 endonuclease (dCas9), which is fused to Poseida’s proprietary dimeric nuclease, Clo051.
Unlike conventional CRISPR-Cas, Clo051 is the scissors in Cas-CLOVER and dCas9 merely serves as a fusion protein that guides the scissors to the correct cut site. The technology can be used to make deletions, insertions, and site-specific mutations, and it is highly specific. This specificity comes from the fact that Clo051 activity requires dimerisation, where two subunits of Clo051 assemble to form a dimer at the target site, and each subunit is associated with a guide RNA (gRNA).
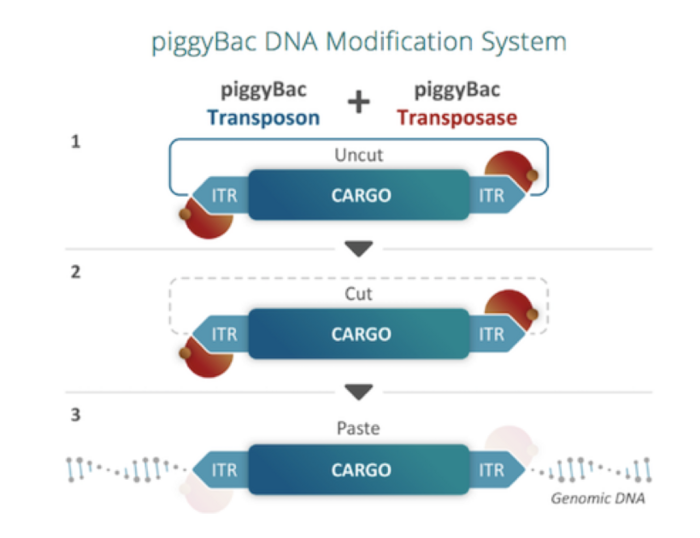
piggyBac cuts and pastes gene-editing cargo from delivery vectors to the genome
piggyBac is a naturally occurring mobile genetic element that effectively "cuts and pastes" or transposes DNA between vectors and chromosomes.
When Eric Ostertag founded Poseida Therapeutics as a spinout of parent genetic engineering company Transposagen Biopharmaceuticals in 2014, he saw huge potential for piggyBac in human gene-editing applications, as he explains:
»It’s cheap and easy to produce, it doesn’t require host proteins to function and there are virtually no cargo size limitations. We can easily load it with 100 kb of gene-editing cargo DNA. Once inside cells, piggyBac efficiently delivers the cargo to every cell type regardless of whether or not the cell is dividing, and it is safe. We generally see between 1 and 3 piggyBac integrations per clone and with sequencing we can determine where these integrations occur. We have never seen piggyBac inactivate a tumour suppressor gene or turn on an oncogene.«
A transposase enzyme recognises inverted terminal repeat sequences (ITRs) on either end of the piggyBac transposon and allows its specific integration at TTAA sites that are randomly dispersed throughout the genome (Figure 2).
piggyBac preferentially integrates into TTAA sites but Poseida is working to make the system 100% tunable, by engineering it to specifically integrate into designated safe-harbour sites.
Unique approach to correcting genetic diseases in children
Before piggyBac can integrate gene-editing cargo sequences into the genome, piggyBac itself must be delivered to cells. This may be done in a number of ways including electroporation, adeno-associated virus (AAV) and non-viral methods such as biodegradable nanoparticles depending on whether the application is ex vivo or in vivo.
The need for this ‘standard’ delivery step used in other gene-editing programmes begs the question of why include the piggyBac step at all if it’s still necessary to use a virus or nanoparticles to get piggyBac and its cargo into cells. Eric Ostertag tells us that the answer lies in the integrative nature of piggyBac, which may also hold a key to correcting genetic diseases in children:
»In contrast to the majority of other gene-editing approaches, piggyBac integrates into the genome to allow constitutive gene delivery. When the therapeutic transgene doesn’t integrate it will be eventually be lost from the cell through cell division. Let’s consider a gene therapy for a liver disease in adults. Cell division in the adult liver is very slow and adults tend to have less severe disease compared to children, so transient expression from the therapeutic transgene may be effective. However, transient gene therapy is problematic in tissues with a high cell turnover, e.g., in rapidly dividing paediatric liver cells.«
Thus, besides its CAR T programmes, Poseida is also harnessing the integrative properties of piggyBac to develop cures for congenital liver diseases in children. The idea is that piggyBac can be used to stably integrate therapeutic transgenes into the hepatocyte genome so that they persist even when the cells divide. The company has programmes at the pre-clinical stage for Ornithine Transcarbamylase (OTC) Deficiency and Haemophilia A, both severe congenital diseases that implicate the liver.
»All congenital diseases manifest in children and the most severely affected people are affected earlier in life. We believe that the combination of AAV and piggyBac or nanoparticle and piggyBac is the only technology that will effectively treat the paediatric patient population.«, said Eric Ostertag.
“All congenital diseases manifest in children and the most severely affected people are affected earlier in life. We believe that the combination of AAV and piggyBac or nanoparticle and piggyBac is the only technology that will effectively treat the paediatric patient population.”Dr. Eric Ostertag
Poseida’s allogeneic CAR T candidates may overtake their autologous counterparts
Within the CAR T space, the pursuit for off-the-shelf therapies is fast becoming the norm, for reasons such as unlimited and consistent production, faster time to treatment, and better overall fitness of donor cells, to name a few.
In spite of promising clinical results so far for the autologous CAR T candidates in liquid and solid tumours, Poseida is also pursuing allogeneic CAR Ts and if all goes well these will likely overtake the company’s autologous candidates.
»We have two allogeneic programmes approaching the clinic, with P-BCMA-ALLO1 for multiple myeloma and P-MUC1C-ALLO1, a pan-specific candidate that targets many solid cancer types. We have demonstrated the elimination of tumour cells to undetectable levels in two pre-clinical models of breast cancer and a preclinical model of ovarian cancer, and we believe that the fully allogeneic CAR T product candidates will actually work better than their autologous counterparts. If these continue to look good, they will likely take priority over the two autologous CAR Ts that we already have in the clinic,« explains Eric Ostertag.
Last week, Poseida received clearance from the FDA to proceed with a clinical trial for P-BCMA-ALLO1 for multiple myeloma, and it expects to dose the first patients later this year and the first patients with the pan-solid CAR T candidate early next year.
Increased stemness is key to effective allogeneic CAR T cell therapies
Poseida's allogeneic CAR T candidates are moving steadily forward, but what makes them stand out? It turns out that piggyBac has another fortuitous property, as Eric Ostertag tells us:
»Another major advantage of piggyBac is that it works to deliver its therapeutic transgene in resting T cells, which is unlike viral-based vectors. This allows us to obtain high percentages of desirable stem cell memory T cells (TSCM) in our products. These percentages go up even more when we go from patient- to healthy donor-derived cells in our fully allogeneic CAR T product candidates.«
The stemness in Poseida’s allogeneic products is reported to be between 60-80%, and in its most predictive animal models, this high percentage of TSCM increases efficacy as well as the longevity of the edited cells.
Working with such a high percentage of TSCM also helps the company to circumvent the catch 22 situation faced in other allogeneic CAR T programmes. That is, the necessity to gene edit the cells, for example to remove the endogenous T cell receptor (TCR) to prevent graft-vs.-host disease, which may be fatal. However, genetic manipulation and expansion outside of the body often decreases the performance of the cells when put back into a patient, a problem that some in the field have dubbed the “allo tax.” Typical CAR T manufacturing methods require activation of the T cells. Once the cells are activated and start dividing, they also start differentiating, losing their desirable stemness and becoming exhausted. Poseida’s Cas-CLOVER gene-editing system, like piggyBac, works in resting T cells, allowing for the maintenance of a very high percentage of TSCM cells all the way through manufacturing.
A second problem is that genetic editing of the endogenous TCR eliminates a major mechanism used to increase the number of cells during manufacturing, so most allogeneic CAR T manufacturing methods have low yield and therefore cannot product a large number of doses. This is a significant dilemma within the allogeneic CAR T field, but Poseida has a unique solution:
»Gene editing of the endogenous TCR limits the number of doses than can be generated at once, and most companies in the allogeneic CAR T field are not getting more than a dozen doses per manufacturing run. At Poseida, we use a proprietary booster molecule to transiently replace the function of TCR during the manufacturing process. This is not a normal endogenous TCR but it allows significant cell expansion while maintaining stemness, so we can make hundreds of off-the-shelf doses in a single run while keeping the high level of stemness,« says Eric Ostertag.
A safety and efficacy profile that may render autologous CAR T extinct
Poseida’s CAR T candidates also have a safety edge that allows fully outpatient treatment. This is caused by the fact that TSCM takes a little bit longer to differentiate into effector cells compared with products comprised of mostly differentiated cells to begin with.
Eric Ostertag compares Poseida’s CAR T candidates to pro drugs: »While our competitors are making the actual drug i.e., the effector cells, we're putting in a pro drug that takes about a week longer to reach peak expansion, or C Max – this is where the effector cells come out of the bone marrow and attack the cancer.«
In the approximately 100 patients dosed in their P-BCMA-101 clinical trial so far, Poseida has never observed a grade three or higher cytokine release syndrome (CRS) event, never had a patient go to the ICU, and never encountered patient death due to a side effect.
»This allows fully outpatient dosing, and that is unique. I'm not aware of any other company that can do that,« says Eric Ostertag.
The future is looking bright for Poseida and if Eric Ostertag is right, Poseida’s allogeneic CAR T candidates are set to be a game changer within gene therapy:
»We believe that our fully allogeneic products will be more efficacious, more persistent leading to more durable responses, and will maintain the potentially best-in-class tolerability profile that we are already seeing in clinical studies. And if we have all of this, plus the ability to make hundreds of doses in a single manufacturing run, the cost of manufacturing per dose will drop by as much as 100x. At that point, autologous CAR T is pretty much extinct.«
In June 2021, CRISPR Medicine News interviewed Jack Crawford, CEO of Demeetra AgBio (Kentucky), a sister company of Poseida Therapeutics. Demeetra is using Cas-CLOVER to develop plant-based drugs and to enhance bioprocessing for human and animal health. You can read more about how Cas-CLOVER works and how it compares to conventional CRISPR in our interview with Jack Crawford.
Tags
ArticleInterviewNewsin vivoNon-viralAdeno-associated virus (AAV)Multiple Myeloma, MMProstate CancerSolid Tumor AdultSolid TumoursCancerRare DiseaseCAR-TCas-CLOVERPoseida TherapeuticsClinical
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.