Highlight: CRISPR reduces the cause of blindness in monkeys but comes with toxicity
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
Glenn Yiu, a Professor of Ophthalmology, and his team at the University of California, Davis, used adeno-associated viral (AAV) vectors to deliver CRISPR-Cas9 and guide RNA (gRNA) targeting a conserved region of the VEGFA gene. The study, recently published in Molecular Therapy, aimed to achieve long-term suppression of VEGF, a key factor in abnormal blood vessel growth, which is a hallmark of choroidal neovascularisation (CNV).
»Given the burden of repeated eye injections for patients with wet age-related macular degeneration (AMD), the prospect of a one-time treatment is very exciting, although additional work may be needed to understand the mechansim of the retinal disruption we observed in our study,« Glenn Yiu says to CRISPR Medicine News.
Due to the size limitations of the AAV8 vector, the researchers utilised a dual-vector approach, with one AAV carrying Cas9 and the other the gRNA. Cas9 and gRNA vectors were sub-retinally injected at a 1:1 ratio into both eyes of four animals, with active and empty gRNA vectors administered to the contralateral eyes of each animal (see Figure 1).
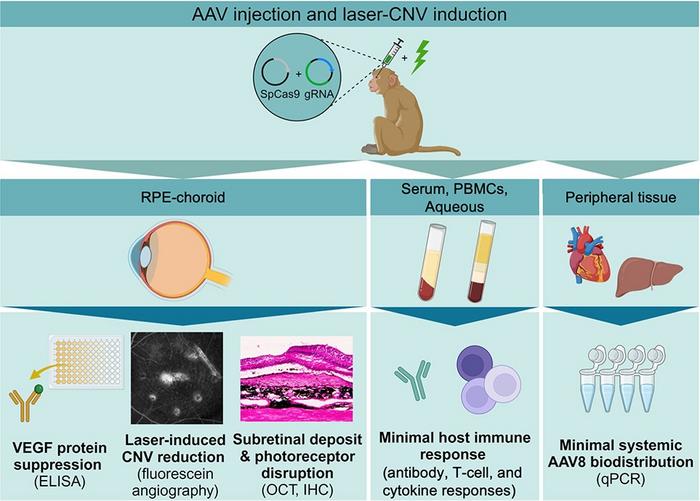
CRISPR-mediated VEGFA ablation showed promise in reducing VEGF levels, particularly in eyes treated with low (6 x 1011 vector genomes/eye) AAV doses, where VEGF protein concentrations dropped by 33%. This reduction led to a corresponding decrease in CNV severity, as demonstrated by fluorescein angiography (FA) imaging of laser-induced lesions in the eyes.
“While we don't know the exact mechanism for the retinal damage, we believe it is related to the high Cas9 expression as we have not observed toxicity using AAVs expressing other transgenes”Glenn Yiu
However, the high (6 x 1012 vector genomes/eye) AAV dose resulted in significant retinal toxicity, which appeared to mask the therapeutic effects. Retinal imaging revealed the presence of subfoveal deposits and concentric macular rings, which corresponded to outer retinal thinning, especially in the photoreceptor layer. These disruptions were confirmed by histological analysis, which identified sub-retinal fibrosis marked by the presence of retinal pigment epithelial (RPE) cells and fibrotic materials including collagen and vimentin.
Interestingly, eyes that received the higher AAV dose showed consistently low VEGF levels, regardless of whether they were treated with active or empty gRNA. This suggests that higher level of retinal disruption may have damaged a larger number of VEGF-producing cells (see Figure 2).
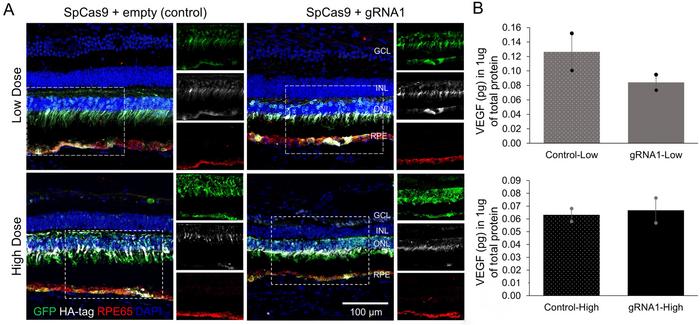
The retinal damage appeared to be unrelated to VEGFA gene editing itself, as similar effects were observed in control eyes that received AAV vectors without the active gRNA. These findings indicate that the damage was likely due to the high AAV vector dose or elevated Cas9 expression in retinal cells.
»While we don't know the exact mechanism for the retinal damage, we believe it is related to the high Cas9 expression as we have not observed toxicity using AAVs expressing other transgenes. Because SpCas9 is a bacterial protein, high expression even within an "immune-privileged" space like the inside of the eye could potentially trigger local inflammation,« says Glenn Yiu.
Immunostaining showed activation of glial and microglial cells, suggesting local inflammation, while systemic immune responses remained minimal. The animals did not show significant humoral or cellular immune reactions against the AAV vector or the Cas9 protein. However, local inflammation markers, particularly CCL2 and CXCL10, were elevated in the treated eyes.
The study highlights the potential of CRISPR-Cas9 for long-term VEGF suppression and CNV treatment, while stressing the need to optimise the AAV delivery system to minimise retinal damage. Lower vector doses or alternative delivery methods could offer safer options for future therapeutic strategies.
Tzu-Ni Sin and Glenn Yiu from the University of California, Davis, led the study, which was published online in Molecular Therapy on September 27, 2024.
An unrelated French study, published last week in Molecular Therapy Nucleic Acids, also showed retinal toxicity related to high Cas9 concentrations.
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleCMN BriefsCMN HighlightsNewsDeliveryRibonucleoprotein (RNP)Adeno-associated virus (AAV)Cas9
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







