CRISPR Medicine in 2024 - A Recap
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
CRISPR Medicine News in 2024
In January, we kicked off our brand-new CMN Live interview series. In our inaugural episode, which took place shortly after CASGEVY's first approvals, we were joined by Eric Kmiec, Director of ChristianaCare Gene Editing Institute and CEO at CorriXR Therapeutics. We discussed both the field's remarkable progress in therapeutic gene editing and the challenges that lie ahead. You can read a write-up of our discussion here.
With the support of the CRISPR medicine community, we successfully launched the CRISPR Medicine Conference in Copenhagen in April 2024. The inaugural event drew more than 400 academic, industrial, and clinical researchers, clinicians, and industry professionals from around the world to discuss research and clinical advances in the therapeutic gene-editing field. We had barely recovered from CRISPRMED24 when planning for CRISPRMED25 got underway!
With just under 3 months to go until CRISPRMED25, which kicks off with a virtual event on the 7th of April, we are excited to once again host some of the world's leading scientists in Copenhagen. Check out our preliminary programme and view our speaker list here.
With an incredible line-up of invited speakers and compelling panel discussions covering the history of genome editing, the regulatory landscape for gene-editing therapeutics, and patient access to CRISPR medicine, CRISPRMED25 promises to further advance our understanding of gene-editing technology and its transformative potential in medicine.
Following requests from our readers, September 2024 saw the revival of our sister publication CARBON - which had initially launched as a pilot project in 2022 - bringing you a monthly newsletter with all your CRISPR AGBio News.
Looking ahead, we are very excited to be hosting the CRISPR Medicine Conference 2025 in just three months time and we look forward to seeing what 2025 has in store for the therapeutic gene-editing field!
Clinical progress and global approvals for CASGEVY
As of today, CRISPR Medicine News has included 239 clinical trials involving a gene-editing or gene-edited therapeutic candidate in its manually-curated clinical trial register. Of these, 152 trials are active, including recruiting, not yet recruiting, and enroling by invitation (see Chart 1 for a full status breakdown of all 239 trials).
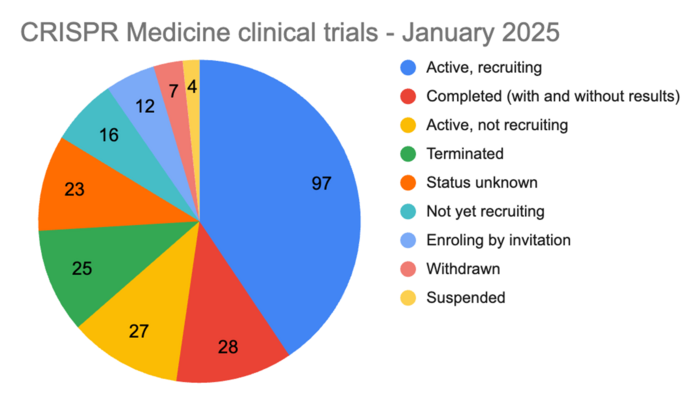
In 2024, therapeutic candidates for blood and solid cancers dominated the clinical gene-editing landscape, making up almost half of all trials with blood cancers alone accounting for almost a third of all trials.
Within the rare disease space, gene-editing therapeutic candidates are now in the clinic for a range of conditions (see Chart 2 for a full disease category breakdown), including metabolic disorders, immunodeficiencies, inherited eye diseases, neurological conditions and others.
Gene-editing strategies to treat or cure haemoglobinopathies continue to lead the way with 5 out of 9 Phase 3 clinical trials evaluating a gene-editing candidate for sickle cell disease and/or beta thalassemia. With additional Phase 3 trials now ongoing in hereditary amyloidosis and immunodeficiencies including Intellia Therapeutics' Phase 3 trial in hereditary angioedema, we hope to see the next wave of gene-editing approvals in the coming years.
Following the UK's landmark approval of CASGEVY for the treatment of sickle cell disease and beta thalassemia in November 2023, Bahrain's approval in December 2023, and the FDA's approval for sickle cell disease in December 2023, early 2024 saw additional approvals for CASGEVY. Beta thalassemia was added to the FDA's list of approved indications in in January 2024, while Saudi Arabia's regulatory agency and the European Medicines Agency approved CASGEVY for the treatment of sickle cell disease and beta thalassemia in January and February 2024, respectively. CASGEVY was also approved for both conditions in Canada in September 2024.
Advances in in vivo genome editing
2024 was a great year for in vivo genome editing, with clinical progression of the first epigenome silencer to cure chronic hepatitis B virus, advances in our understanding of the heritability of epigenome editing in vivo and breakthroughs in precise microbiome editing.

In March 2024, Dr. Brian Cosgrove, a Principal Scientist at Tune Therapeutics, spoke with us about the company’s lead programme, TUNE-401, which is being developed as a "one-and-done" functional cure for chronic hepatitis B virus (HBV). TUNE-401 works by mimicking a rare natural phenomenon whereby some patients develop epigenetic control over the virus. It uses catalytically-dead Cas9 (dCas9) fused to a methyltransferase and another epigenetic repressor to target a conserved “master controller” sequence shared by both covalently closed circular DNA (cccDNA) and integrated viral DNA (intDNA). By delivering this system via lipid nanoparticles, TUNE-401 programmes repressive epigenetic marks, such as DNA methylation and heterochromatin formation, to durably inactivate all forms of the virus, potentially providing a long-lasting functional cure for chronic hepatitis B.
Preclinical data for TUNE-401 showed almost complete repression of HBV RNA, with viral silencing persisting beyond 550 days in in vitro studies. Since our interview with Brian Cosgrove, Tune has received regulatory approval to begin testing TUNE-401 in clinical trials in New Zealand and Hong Kong. TUNE-401 could potentially transform treatment for the estimated 254 million people living with chronic HBV globally, particularly in Asian populations where early-life infection makes disease control challenging.
In April, we interviewed Martino Alfredo Cappelluti at San Raffaele - Telethon Institute for Gene Therapy (SR-Tiget) in Italy about his team's breakthrough study demonstrating successful long-term in vivo epigenetic silencing using a single-dose "hit-and-run" approach. Cappelluti's team and collaborators first conducted in vitro screening of different DNA-binding enzymes and found zinc-finger protein (ZFP)-based engineered transcriptional repressors (ZFN-ETRs) to most efficiently silence the target gene Pcsk9, which regulates cholesterol levels. Using LNP to deliver mRNA encoding the ZFP-based repressor to mice, the team observed a significant reduction in circulating Pcsk9 protein levels that persisted for almost one year. Even after inducing liver regeneration through partial hepatectomy, the decrease in circulating Pcsk9 levels and the levels of CpG methylation at the Pcsk9 promoter were maintained. This suggests that epigenetic modifications can persist through cell division, confirming the heritability of epigenetic Pcsk9 silencing and the potential of this approach for long-term therapeutic effects without the need for continuous treatment.
They further improved their approach with an all-in-one editing construct called EvoETR-8, which reduced circulating Pcsk9 levels by 75%. This is comparable to conventional gene editing but with the advantage that EvoETR-8 doesn’t introduce DNA breaks. While delivery of gene-editing components to organs beyond the liver remains challenging, this proof-of-concept study opens new possibilities for developing therapies based on epigenetic silencing rather than permanent DNA modification.
In July, Eligo Bioscience reported a major first in microbiome editing, demonstrating that it is possible to specifically target a single bacterial strain in the gut of a living mouse. Using an engineered phage-derived capsid to deliver a base editor to the mouse gut, Eligo scientists achieved nearly 100% base-editing efficiency when targeting a single bacterial target strain among hundreds of species present in the microbiome. Their findings, published in Nature, represent a significant improvement over previous attempts to edit the microbiome, which have been limited by poor delivery, limited targeting range or low editing efficiency. Eligo’s scientists could disrupt antibiotic-resistance genes and virulence factors with extreme precision, and edits remained stable for at least 42 days following a single oral treatment.
This milestone opens up the possibility to selectively modify bacterial genes that drive disease or influence the outcome of certain therapies while preserving overall microbiome composition and function. In September 2024, we followed up with a CMN Live interview with Xavier Duportet to discuss the human microbiome, its role in health and disease, and how gene editing within the microbiome could transform medicine.
Don't miss the next CMN Live episode!
Sign up to attend our next CMN Live episode with Associate Professor Alessia Cavazza, UCL, here.
Find all CMN Live episodes here.

Gene editing continues to revolutionise cancer research
In 2024, CRISPR technology delivered several breakthroughs in our understanding of cancer and paved the way for precision medicine. Continuous advances in CRISPR screening technologies are catalysing large studies to identify new genetic drivers of cancer metastasis, which is the primary cause of death in many cancers, for example, prostate cancer. At the beginning of the year, three research groups independently used genome-wide CRISPR screens to identify three crucial genes - CITED2, KMT2C, and PRMT7 - that drive metastasis in this disease
In a conversation with Juan Martín Arriaga at Icahn School of Medicine at Mount Sinai and Martin Kristian Thomsen at Aarhus University – who characterised CITED2 and KMT2C, respectively – they explained how these genes work in very different ways. CITED2 functions as a transcriptional regulator with a dual role depending on the tumour context. Initially, it can inhibit the transcription factor E2F1, preventing metastasis in primary tumours. However, as the tumour evolves or in specific cell subtypes, CITED2 may switch to upregulating E2F1, driving tumour proliferation and metastasis. This complex behaviour underscores the importance of its interaction with E2F1 rather than its overall expression levels.
In contrast, KMT2C and PRMT7 (the latter characterised by a research group from the Complutense University of Madrid) are involved in epigenetic regulation. KMT2C encodes a histone methyltransferase that modifies chromatin structure, influencing gene expression. Its loss leads to overexpression of specific genes, such as Odam and Cabs1, that are highly expressed in metastases, suggesting a crucial role in cancer progression. PRMT7, on the other hand, catalyses arginine methylation in histones, affecting gene expression through both activating and repressive marks. Inhibition of PRMT7 significantly reduced the invasive capacity of human prostate cancer cell lines both in vitro and in vivo in chicken embryo and mouse xenograft models. Together, these findings emphasise the therapeutic and diagnostic potential of targeting the three genes or their pathways to combat prostate cancer metastasis.
Olivier Belli and colleagues at ETH Zürich took another approach, pushing the boundaries of genetic mapping by combining base- and prime editing to explore the mutational landscape of the EGFR gene, which is a critical player in many cancers. Mutations in the receptor’s intracellular C-terminal tail were found to disrupt splice sites that lead to truncations, and, unexpectedly, this caused constitutive receptor activation and drove drug resistance in some cancer cells. Other mutations were shown to alter receptor activation and downstream signalling, contributing to tumorigenesis and resistance to tyrosine kinase inhibitors. A key takeaway was the discovery that some mutations conferred drug resistance only in cancerous (PC-9) cell lines but not in wild-type (MCF10A) cells, emphasising the importance of secondary mutations in advanced cancers. These findings illustrate how genetic changes influence cancer progression and drug response, depending on the mutation and cellular context.
In an interview with Fiona Behan at GlaxoSmithKline, we learned about how synthetic lethality has been harnessed through combinatorial CRISPR screening, revealing cancer-specific gene dependencies. By exploring interactions between gene pairs, her team identified vulnerabilities unique to cancer cells. With the aid of machine learning, this approach becomes even more powerful, narrowing the search for promising therapeutic targets. However, rather than pursuing CRISPR-based therapies, Fiona Behan focuses on validating these targets for small-molecule drug development. She mentions employing CRISPR inhibition tools to suppress gene expression and exploring inducible CRISPR systems that can mimic the effects of small molecules, enabling precise control of gene activity during validation. This approach aligns with GSK’s strategic shift away from cell and gene therapies towards oligonucleotide and small-molecule-based treatments.
In immunotherapy, Shipra Das and Laurent Poirot at Cellectis Inc. is taking a groundbreaking approach to enhance the potential of CAR-T cells for treating solid tumours. In that study, the team at Cellectis used TALEN technology to engineer CAR-T cells to incorporate an IF/THEN logic gate that activates the therapy only in the presence of specific tumour conditions, reducing off-target effects and improving safety. These dual CAR-T cells have demonstrated significant tumour reduction in preclinical models, offering a promising strategy to tackle solid tumours, a challenging frontier in cancer therapy. Cellectis plans to move its TALEN-edited dual CAR-T cell therapy into clinical studies within two to five years, following extensive preclinical validation to ensure safety and efficacy. Their approach includes testing in more physiologically relevant models to address the complexity of solid tumours. Read our interview with Das and Poirot here.
These studies highlight CRISPR’s and TALEN’s transformative role in cancer research by enabling unprecedented precision in identifying genetic vulnerabilities, mapping complex mutational landscapes, and engineering advanced therapeutic strategies like dual CAR-T systems. Together, they demonstrate how gene-editing tools are driving a shift toward personalised, targeted treatments that address the complexities of cancer genomes while minimising off-target effects.
Advances in CRISPR delivery open new possibilities in gene editing
Efficient delivery of CRISPR tools remains a critical challenge in gene editing, especially for in vivo applications targeting hard-to-reach organs like the lungs and brain. However, recent advancements in delivery technologies are paving the way for more effective, safe, and tissue-specific treatments. We started 2024 out with a recap of current CRISPR delivery methods and challenges, but during the year, we revisited the topic several times.
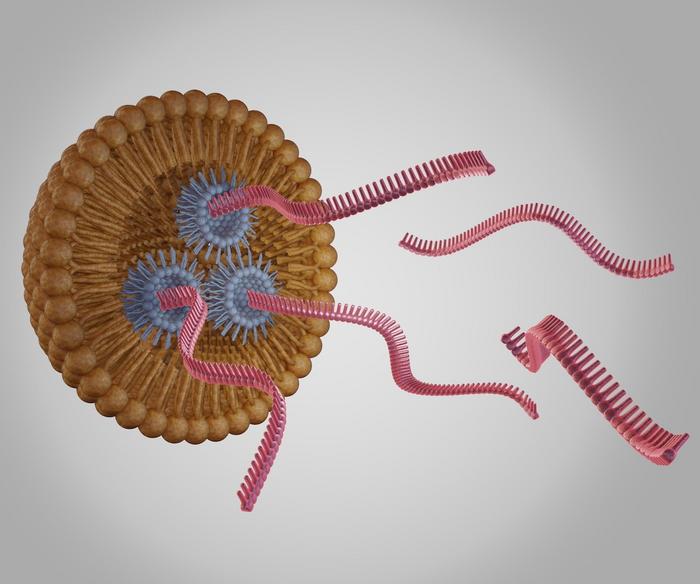
One promising approach for targeted delivery comes from researchers at Johns Hopkins University, who have developed engineered poly(beta-amino ester) (PBAE) nanoparticles for systemic mRNA delivery to lung cells. Unlike traditional methods, which rely on inhalation or intratracheal administration and often fail to penetrate mucus-covered airways, these biodegradable nanoparticles effectively target lung tissues after intravenous administration. Preclinical models demonstrated high transfection rates and minimal toxicity, with researchers achieving up to 90% gene-editing efficiency in human airway cells. This system holds particular promise for multi-organ diseases such as cystic fibrosis, where systemic delivery is essential.
While targeting the lungs addresses a critical need, delivering CRISPR tools to the brain poses even greater challenges because of the protective blood-brain barrier. Researchers at the University of California have developed acid-degradable PEGylated lipid nanoparticles (ADP-LNPs) that can deliver CRISPR-based mRNA tools to embryonic brain cells during in utero development. These nanoparticles achieved widespread genetic modifications across the brain in mouse models, with minimal inflammation or toxicity. The potential to intervene prenatally in neurodevelopmental disorders such as Angelman syndrome marks a revolutionary step in genetic medicine.
In our inaugural CMN live interview, Eric Kmiec at the ChristianaCare Gene Editing Institute highlighted that while viral vectors have been successfully used in gene editing applications, they carry the risk of off-target mutagenesis due to prolonged expression of editing components. Non-viral delivery methods, such as lipid nanoparticles (LNPs), offer the possibility for transient editing and have shown promise in targeting liver-based diseases, as evidenced by recent successes from Intellia Therapeutics and Verve Therapeutics. Looking ahead, Eric Kmiec is optimistic about advancements in LNPs for tissue-specific targeting and penetration, which could eventually revolutionise treatments for solid tumours and other difficult-to-treat diseases.
The future looks bright for CRISPR in 2025
The year 2024 highlighted significant advancements in CRISPR medicine, with progress spanning cancer research, in vivo genome editing, and clinical trials. Notably, the approval and expansion of CASGEVY for sickle cell disease and beta-thalassemia marked a pivotal step in translating CRISPR-based therapies into practice, with Phase 3 trials for hereditary amyloidosis and hereditary angioedema signalling a broader therapeutic reach. Concurrently, breakthroughs in epigenetic silencing, such as TUNE-401 for chronic hepatitis B and ZFP-based repressors for Pcsk9 silencing, showcased the potential for durable, non-invasive treatments that avoid permanent DNA modifications. These clinical and preclinical successes highlight CRISPR’s ability to address unmet medical needs with unprecedented precision.
Innovations in delivery systems also played a crucial role, with biodegradable nanoparticles enabling targeted lung delivery and acid-degradable lipid nanoparticles facilitating gene editing in the brain. These advances in delivery technology, coupled with the increasing diversity of clinical trials across rare diseases, cancers, and metabolic disorders, underscore the growing impact of gene-editing technologies in medicine. As clinical trials expand and delivery methods become more efficient, gene-editing therapies are poised to redefine treatment landscapes, bringing tailored, long-lasting solutions closer to patients worldwide.
To get more CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
CARBON will bring you the latest news on how CRISPR can shape agriculture for the future to guarantee food security in times of population growth and climate change. To get more CRISPR AgroBio News delivered to your inbox, sign up to the free monthly CARBON Newsletter here.
Tags
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







